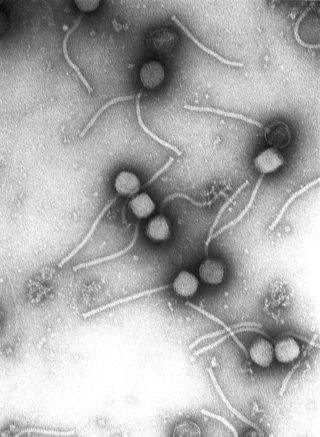
Virology is the scientific study of biological viruses. It is a subfield of microbiology that focuses on their detection, structure, classification and evolution, their methods of infection and exploitation of host cells for reproduction, their interaction with host organism physiology and immunity, the diseases they cause, the techniques to isolate and culture them, and their use in research and therapy.

HIV tests are used to detect the presence of the human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), in serum, saliva, or urine. Such tests may detect antibodies, antigens, or RNA.

In immunology, seroconversion is the development of specific antibodies in the blood serum as a result of infection or immunization, including vaccination. During infection or immunization, antigens enter the blood, and the immune system begins to produce antibodies in response. Before seroconversion, the antigen itself may or may not be detectable, but the antibody is absent. During seroconversion, the antibody is present but not yet detectable. After seroconversion, the antibody is detectable by standard techniques and remains detectable unless the individual seroreverts, in a phenomenon called seroreversion, or loss of antibody detectability, which can occur due to weakening of the immune system or decreasing antibody concentrations over time. Seroconversion refers the production of specific antibodies against specific antigens, meaning that a single infection could cause multiple waves of seroconversion against different antigens. Similarly, a single antigen could cause multiple waves of seroconversion with different classes of antibodies. For example, most antigens prompt seroconversion for the IgM class of antibodies first, and subsequently the IgG class.
Serology is the scientific study of serum and other body fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection, against other foreign proteins, or to one's own proteins. In either case, the procedure is simple.
The hemagglutination assay or haemagglutination assay (HA) and the hemagglutination inhibition assay were developed in 1941–42 by American virologist George Hirst as methods for quantifying the relative concentration of viruses, bacteria, or antibodies.
Heterophile antibodies are antibodies induced by external antigens.

Influenza C virus is the only species in the genus Gammainfluenzavirus, in the virus family Orthomyxoviridae, which like other influenza viruses, causes influenza.
Porcine parvovirus (PPV), a virus in the species Ungulate protoparvovirus 1 of genus Protoparvovirus in the virus family Parvoviridae, causes reproductive failure of swine characterized by embryonic and fetal infection and death, usually in the absence of outward maternal clinical signs. The disease develops mainly when seronegative dams are exposed oronasally to the virus anytime during about the first half of gestation, and conceptuses are subsequently infected transplacentally before they become immunocompetent. There is no definitive evidence that infection of swine other than during gestation is of any clinical or economic significance. The virus is ubiquitous among swine throughout the world and is enzootic in most herds that have been tested. Diagnostic surveys have indicated that PPV is the major infectious cause of embryonic and fetal death. In addition to its direct causal role in reproductive failure, PPV can potentiate the effects of porcine circovirus type II (PCV2) infection in the clinical course of postweaning multisystemic wasting syndrome (PMWS).

Psittacine beak and feather disease (PBFD) is a viral disease affecting all Old World and New World parrots. The causative virus—beak and feather disease virus (BFDV)—belongs to the taxonomic genus Circovirus, family Circoviridae. It attacks the feather follicles and the beak and claw matrices of the bird, causing progressive feather, claw and beak malformation and necrosis. In later stages of the disease, feather shaft constriction occurs, hampering development until eventually all feather growth stops. It occurs in an acutely fatal form and a chronic form.

Bovine viral diarrhea (BVD), bovine viral diarrhoea or mucosal disease, previously referred to as bovine virus diarrhea (BVD), is an economically significant disease of cattle that is found in the majority of countries throughout the world. Worldwide reviews of the economically assessed production losses and intervention programs incurred by BVD infection have been published. The causative agent, bovine viral diarrhea virus (BVDV), is a member of the genus Pestivirus of the family Flaviviridae.
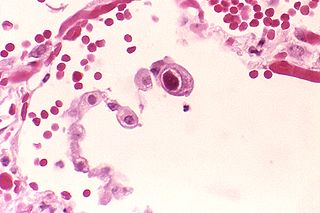
Human betaherpesvirus 5, also called human cytomegalovirus (HCMV), is species of virus in the genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae or herpesviruses. It is also commonly called CMV. Within Herpesviridae, HCMV belongs to the Betaherpesvirinae subfamily, which also includes cytomegaloviruses from other mammals. CMV is a double-stranded DNA virus.
Bovine leukemia virus (BLV) is a retrovirus which causes enzootic bovine leukosis in cattle. It is closely related to the human T‑lymphotropic virus type 1 (HTLV-I). BLV may integrate into the genomic DNA of B‑lymphocytes as a DNA intermediate, or exist as unintegrated circular or linear forms. Besides structural and enzymatic genes required for virion production, BLV expresses the Tax protein and microRNAs involved in cell proliferation and oncogenesis. In cattle, most infected animals are asymptomatic; leukemia is rare, but lymphoproliferation is more frequent (30%).

Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms begin from one to four days after exposure to the virus and last for about 2–8 days. Diarrhea and vomiting can occur, particularly in children. Influenza may progress to pneumonia, which can be caused by the virus or by a subsequent bacterial infection. Other complications of infection include acute respiratory distress syndrome, meningitis, encephalitis, and worsening of pre-existing health problems such as asthma and cardiovascular disease.

In molecular biology, hemagglutinins are receptor-binding membrane fusion glycoproteins produced by viruses in the Paramyxoviridae and Orthomyxoviridae families. Hemagglutinins are responsible for binding to receptors on red blood cells to initiate viral attachment and infection. The agglutination of red cells occurs when antibodies on one cell bind to those on others, causing amorphous aggregates of clumped cells.
Virus quantification is counting or calculating the number of virus particles (virions) in a sample to determine the virus concentration. It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines, recombinant proteins using viral vectors, and viral antigens all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.
The plaque reduction neutralization test is used to quantify the titer of neutralizing antibody for a virus.
Avian orthoreovirus, also known as avian reovirus, is an orthoreovirus from the Reoviridae family. Infection causes arthritis and tenosynovitis in poultry. It can also cause respiratory disease.

Surround optical-fiber immunoassay (SOFIA) is an ultrasensitive, in vitro diagnostic platform incorporating a surround optical-fiber assembly that captures fluorescence emissions from an entire sample. The technology's defining characteristics are its extremely high limit of detection, sensitivity, and dynamic range. SOFIA's sensitivity is measured at the attogram level (10−18 g), making it about one billion times more sensitive than conventional diagnostic techniques. Based on its enhanced dynamic range, SOFIA is able to discriminate levels of analyte in a sample over 10 orders of magnitude, facilitating accurate titering.

George Keble Hirst, M.D. was an American virologist and science administrator who was among the first to study the molecular biology and genetics of animal viruses, especially influenza virus. He directed the Public Health Research Institute in New York City (1956–1981), and was also the founding editor-in-chief of Virology, the first English-language journal to focus on viruses. He is particularly known for inventing the hemagglutination assay, a simple method for quantifying viruses, and adapting it into the hemagglutination inhibition assay, which measures virus-specific antibodies in serum. He was the first to discover that viruses can contain enzymes, and the first to propose that virus genomes can consist of discontinuous segments. The New York Times described him as "a pioneer in molecular virology."
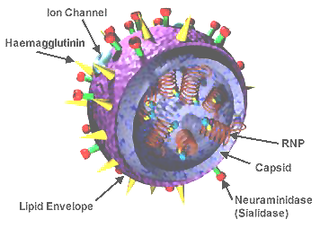
Influenza D virus is a species in the virus genus Deltainfluenzavirus, in the family Orthomyxoviridae, that causes influenza.














