Related Research Articles

Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc).

An amylase is an enzyme that catalyses the hydrolysis of starch into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase. Specific amylase proteins are designated by different Greek letters. All amylases are glycoside hydrolases and act on α-1,4-glycosidic bonds.

Diphosphate—fructose-6-phosphate 1-phosphotransferase also known as PFP is an enzyme of carbohydrate metabolism in plants and some bacteria. The enzyme catalyses the reversible interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate using inorganic pyrophosphate as the phosphoryl donor:

Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch and glycogen. Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds.

Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose.

Pullulanase is a specific kind of glucanase, an amylolytic exoenzyme, that degrades pullulan. It is produced as an extracellular, cell surface-anchored lipoprotein by Gram-negative bacteria of the genus Klebsiella. Type I pullulanases specifically attack α-1,6 linkages, while type II pullulanases are also able to hydrolyse α-1,4 linkages. It is also produced by some other bacteria and archaea. Pullulanase is used as a processing aid in grain processing biotechnology.

Brown rice (malt) syrup, also known as rice syrup or rice malt, is a sweetener which is rich in compounds categorized as sugars and is derived by steeping cooked rice starch with saccharifying enzymes to break down the starches, followed by straining off the liquid and reducing it by evaporative heating until the desired consistency is reached. The enzymes used in the saccharification step are supplied by an addition of sprouted barley grains to the rice starch or by adding bacterial- or fungal-derived purified enzyme isolates.
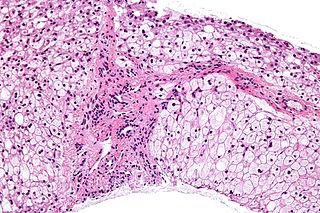
Glycogen storage disease type III (GSD III) is an autosomal recessive metabolic disorder and inborn error of metabolism (specifically of carbohydrates) characterized by a deficiency in glycogen debranching enzymes. It is also known as Cori's disease in honor of the 1947 Nobel laureates Carl Cori and Gerty Cori. Other names include Forbes disease in honor of clinician Gilbert Burnett Forbes (1915–2003), an American physician who further described the features of the disorder, or limit dextrinosis, due to the limit dextrin-like structures in cytosol. Limit dextrin is the remaining polymer produced after hydrolysis of glycogen. Without glycogen debranching enzymes to further convert these branched glycogen polymers to glucose, limit dextrinosis abnormally accumulates in the cytoplasm.
Isomaltase is an enzyme that breaks the bonds linking saccharides, which cannot be broken by amylase or maltase. It digests polysaccharides at the alpha 1-6 linkages. Its substrate, alpha-limit dextrin, is a product of amylopectin digestion that retains its 1-6 linkage. The product of the enzymatic digestion of alpha-limit dextrin by isomaltase is maltose.

β-Amylase is an enzyme with the systematic name 4-α-D-glucan maltohydrolase. It catalyses the following reaction:

α-Amylase is an enzyme that hydrolyses α bonds of large, α-linked polysaccharides, such as starch and glycogen, yielding shorter chains thereof, dextrins, and maltose:
In enzymology, a dextrin dextranase is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-alpha-glucanotransferase is an enzyme that catalyzes a chemical reaction that transfers a segment of a 1,4-alpha-D-glucan to a new position in an acceptor carbohydrate, which may be glucose or a 1,4-alpha-D-glucan.

α-Glucans (alpha-glucans) are polysaccharides of D-glucose monomers linked with glycosidic bonds of the alpha form. α-Glucans use cofactors in a cofactor site in order to activate a glucan phosphorylase enzyme. This enzyme causes a reaction that transfers a glucosyl portion between orthophosphate and α-I,4-glucan. The position of the cofactors to the active sites on the enzyme are critical to the overall reaction rate thus, any alteration to the cofactor site leads to the disruption of the glucan binding site.
Endo-1,3(4)-β-glucanase -β-D-glucan 3(4)-glucanohydrolase) is an enzyme with systematic name 3(or 4)-β-D-glucan 3(4)-glucanohydrolase. It catalyses the following chemical reaction
Dextranase is an enzyme with systematic name 6-α-D-glucan 6-glucanohydrolase. It catalyses the following chemical reaction
Amylo-α-1,6-glucosidase is an enzyme with systematic name glycogen phosphorylase-limit dextrin 6-α-glucohydrolase. It catalyses the hydrolysis of unsubstituted glucose units in glycogen linked by α(1→6) bonds to α(1→4)glucose chains.
Isoamylase is an enzyme with systematic name glycogen 6-α-D-glucanohydrolase. It catalyses the hydrolysis of (1→6)-α-D-glucosidic branch linkages in glycogen, amylopectin and their β-limit dextrins. It also readily hydrolyses amylopectin.
Glucan endo-1,6-β-glucosidase is an enzyme with systematic name 6-β-D-glucan glucanohydrolase. It catalyses random hydrolysis of (1→6)-linkages in (1→6)-β-D-glucans

Neopullulanase is an enzyme of the alpha-amylase family with systematic name pullulan 4-D-glucanohydrolase (panose-forming). This enzyme principally catalyses the following chemical reaction by cleaving pullulan's alpha-1,4-glucosidic bonds:
References
- ↑ Gordon RW, Manners DJ, Stark JR (1975). "The limit dextrinase of the broad bean (Vicia faba)". Carbohydrate Research. 42: 125–134. doi:10.1016/s0008-6215(00)84105-1.
- ↑ Manners DJ (1997). "Observations on the specificity and nomenclature of starch debranching enzymes". Journal of Applied Glycoscience. 44: 83–85.