Related Research Articles

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

In chemistry, pH, also referred to as acidity or basicity, historically denotes "potential of hydrogen". It is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions are measured to have lower pH values than basic or alkaline solutions.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.
In chemistry, an acid dissociation constant is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction

A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electrode and a reference electrode, and so the pH meter is sometimes referred to as a "potentiometric pH meter". The difference in electrical potential relates to the acidity or pH of the solution. Testing of pH via pH meters (pH-metry) is used in many applications ranging from laboratory experimentation to quality control.
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease.
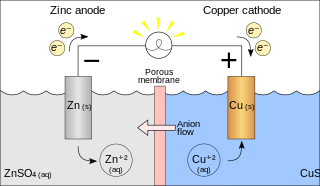
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.
An ion-selective electrode (ISE), also known as a specific ion electrode (SIE), is a transducer that converts the change in the concentration of a specific ion dissolved in a solution into an electrical potential. The voltage is theoretically dependent on the logarithm of the ionic activity, according to the Nernst equation. Ion-selective electrodes are used in analytical chemistry and biochemical/biophysical research, where measurements of ionic concentration in an aqueous solution are required.

Silver chloride is a chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver, which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as a mineral chlorargyrite.
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of pH. The pH electrode is an example of a glass electrode that is sensitive to hydrogen ions. Glass electrodes play an important part in the instrumentation for chemical analysis and physicochemical studies. The voltage of the glass electrode, relative to some reference value, is sensitive to changes in the activity of a certain type of ions.
Amperometric titration refers to a class of titrations in which the equivalence point is determined through measurement of the electric current produced by the titration reaction. It is a form of quantitative analysis.
Redox potential is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is expressed in volts (V). Each species has its own intrinsic redox potential; for example, the more positive the reduction potential, the greater the species' affinity for electrons and tendency to be reduced.

In chemical thermodynamics, isothermal titration calorimetry (ITC) is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules to larger macromolecules in a label-free environment. It consists of two cells which are enclosed in an adiabatic jacket. The compounds to be studied are placed in the sample cell, while the other cell, the reference cell, is used as a control and contains the buffer in which the sample is dissolved.
Total ionic strength adjustment buffer (TISAB) is a buffer solution which increases the ionic strength of a solution to a relatively high level. This is important for potentiometric measurements, including ion selective electrodes, because they measure the activity of the analyte rather than its concentration. TISAB essentially masks minor changes made in the ionic strength of the solution and hence increases the accuracy of the reading.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The four main categories are potentiometry, amperometry, coulometry, and voltammetry.
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant K is expressed as a concentration quotient,
In coordination chemistry, a stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
Binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes.
Equilibrium chemistry is concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid–base, host–guest, metal–complex, solubility, partition, chromatography and redox equilibria.
A ligand binding assay (LBA) is an assay, or an analytic procedure, which relies on the binding of ligand molecules to receptors, antibodies or other macromolecules. A detection method is used to determine the presence and extent of the ligand-receptor complexes formed, and this is usually determined electrochemically or through a fluorescence detection method. This type of analytic test can be used to test for the presence of target molecules in a sample that are known to bind to the receptor.
References
- ↑ D. D. Perrin, B. Dempsey, (1974) Buffers for pH and Metal Ion Control, Chapman and Hall, London, ISBN 978-0-412-21890-3 (Print) 978-94-009-5874-6 (Online).
- ↑ Pretsch, E. (2007) The new wave of ion-selective electrodes. Trac-Trends Anal. Chem. 26, 46-51