
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula CH3OH (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colorless and flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). Methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide.
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences.
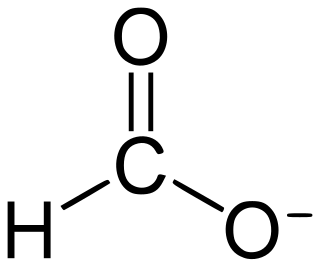
Formate is the conjugate base of formic acid. Formate is an anion or its derivatives such as ester of formic acid. The salts and esters are generally colorless.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leaving group and the methyl group attached to it acts as the electrophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases. Another type of methyl transfer is the radical S-Adenosyl methionine (SAM) which is the methylation of unactivated carbon atoms in primary metabolites, proteins, lipids, and RNA.

The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone.
In enzymology, a 3-demethylubiquinone-9 3-O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an inositol 3-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a protein-glutamate O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a tetrahydromethanopterin S-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-methyl-2-oxobutanoate hydroxymethyltransferase (EC 2.1.2.11) is an enzyme that catalyzes the chemical reaction

The enzyme protein-glutamate methylesterase (EC 3.1.1.61) catalyzes the reaction
In enzymology, a 2-isopropylmalate synthase (EC 2.3.3.13) is an enzyme that catalyzes the chemical reaction

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.
(Methyl-Co methanol-specific corrinoid protein):coenzyme M methyltransferase is an enzyme with systematic name methylated methanol-specific corrinoid protein:coenzyme M methyltransferase. This enzyme catalyses the following chemical reaction
Methylamine-corrinoid protein Co-methyltransferase is an enzyme with systematic name monomethylamine:5-hydroxybenzimidazolylcobamide Co-methyltransferase. This enzyme catalyses the following chemical reaction
Dimethylamine-corrinoid protein Co-methyltransferase is an enzyme with systematic name dimethylamine:5-hydroxybenzimidazolylcobamide Co-methyltransferase. This enzyme catalyses the following chemical reaction
Trimethylamine-corrinoid protein Co-methyltransferase is an enzyme with systematic name trimethylamine:5-hydroxybenzimidazolylcobamide Co-methyltransferase. This enzyme catalyses the following chemical reaction
Tetramethylammonium-corrinoid protein Co-methyltransferase is an enzyme with systematic name tetramethylammonium:5-hydroxybenzimidazolylcobamide Co-methyltransferase. This enzyme catalyses the following chemical reaction








