
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus Vitis. Grapes are a non-climacteric type of fruit, generally occurring in clusters.

Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol or polyphenol and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi. Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. As of 2012, there were between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however, the term applies to any phytochemicals that are induced by microbial infection.

Gnetum gnemon is a gymnosperm species of Gnetum, its native area spans from Mizoram and Assam in India down south through Malay Peninsula, Malay Archipelago and the Philippines in southeast Asia to the western Pacific islands. Common names include gnetum, joint fir, two leaf, melinjo, belinjo, bago, and tulip.

Piceatannol is the organic compound with the formula ( 2C6H3)2CH)2. Classified as a stilbenoid and a phenol, it is a white solid, although samples often are yellow owing to impurities.

Phenolic compounds—natural phenol and polyphenols—occur naturally in wine. These include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.

ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.

α-Viniferin is a stilbene trimer. It can be isolated from Caragana chamlagu and from Caragana sinica and from the stem bark of Dryobalanops aromatica. It is also present in relation to resistance to Botrytis cinerea and Plasmopara viticola in Vitis vinifera and Vitis riparia. It has been shown to inhibit acetylcholinesterase.
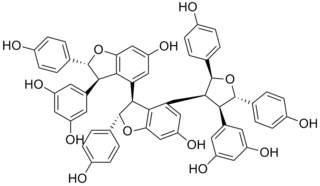
Kobophenol A is a stilbenoid. It is a tetramer of resveratrol. It can be isolated from Caragana chamlagu, from Caragana sinica and from Carex folliculata seeds.

A tetramer (tetra-, "four" + -mer, "parts") is an oligomer formed from four monomers or subunits. The associated property is called tetramery. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol.

Caragana sinica is a species belonging to the genus Caragana.
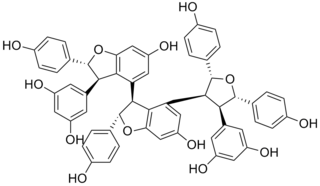
Carasinol B is a stilbenoid tetramer found in Caragana sinica.

Astringin is a stilbenoid, the 3-β-D-glucoside of piceatannol. It can be found in the bark of Picea sitchensis and Picea abies.

δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine leaves. It is also found in plant cell cultures and wine. It can also be found in Rheum maximowiczii.

Isorhapontigenin is a tetrahydroxylated stilbenoid with a methoxy group. It is an isomer of rhapontigenin and an analog of resveratrol. It is found in the Chinese herb Gnetum cleistostachyum, in Gnetum parvifolium and in the seeds of the palm Aiphanes aculeata.

Foeniculoside I is a stilbenoid. It is a glucoside of the stilbene trimer cis-miyabenol C. It can be found in Foeniculi fructus.

















