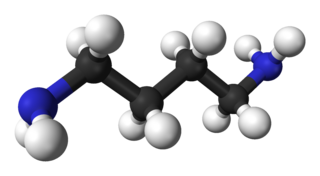
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors.
The enzyme ornithine decarboxylase catalyzes the decarboxylation of ornithine to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer.

Diamine oxidase (DAO), also known "amine oxidase, copper-containing, 1" (AOC1), formerly called histaminase, is an enzyme involved in the metabolism, oxidation, and inactivation of histamine and other polyamines such as putrescine or spermidine. The enzyme belongs to the amine oxidase (copper-containing) (AOC) family of amine oxidase enzymes.
Spermine synthase is an enzyme that converts spermidine into spermine. This enzyme catalyses the following chemical reaction
In enzymology, a spermidine dehydrogenase (EC 1.5.99.6) is an enzyme that catalyzes the chemical reaction
A polyamine oxidase (PAO) is an enzymatic flavoprotein that oxidizes a carbon-nitrogen bond in a secondary amino group of a polyamine donor, using molecular oxygen as an acceptor. The generalized PAO reaction converts three substrates into three products. Different PAOs with varying substrate specificities exist in different organisms. Phylogenetic analyses suggest that PAOs likely evolved once in eukaryotes and diversified by divergent evolution and gene duplication events, though some prokaryotes have acquired PAOs through horizontal gene transfer.
In enzymology, a homospermidine synthase (spermidine-specific) is an enzyme that catalyzes the chemical reaction
In enzymology, a sym-norspermidine synthase is an enzyme that catalyzes the chemical reaction

Flavin-containing amine oxidoreductases are a family of various amine oxidases, including maize polyamine oxidase (PAO), L-amino acid oxidases (LAO) and various flavin containing monoamine oxidases (MAO). The aligned region includes the flavin binding site of these enzymes. In vertebrates, MAO plays an important role in regulating the intracellular levels of amines via their oxidation; these include various neurotransmitters, neurotoxins and trace amines. In lower eukaryotes such as aspergillus and in bacteria the main role of amine oxidases is to provide a source of ammonium. PAOs in plants, bacteria and protozoa oxidise spermidine and spermine to an aminobutyral, diaminopropane and hydrogen peroxide and are involved in the catabolism of polyamines. Other members of this family include tryptophan 2-monooxygenase, putrescine oxidase, corticosteroid-binding proteins, and antibacterial glycoproteins.

Diamine acetyltransferase 1 is an enzyme that in humans is encoded by the SAT1 gene found on the X chromosome.

Spermine oxidase is an enzyme that in humans is encoded by the SMOX gene.
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature.
Carboxynorspermidine synthase (EC 1.5.1.43, carboxynorspermidine dehydrogenase, carboxyspermidine dehydrogenase, CASDH, CANSDH) is an enzyme with systematic name carboxynorspermidine:NADP+ oxidoreductase. This enzyme catalyses the following chemical reactions
N1-acetylpolyamine oxidase (EC 1.5.3.13, hPAO-1, mPAO, hPAO) is an enzyme with systematic name N1-acetylpolyamine:oxygen oxidoreductase (3-acetamidopropanal-forming). This enzyme catalyses the following chemical reaction
N8-acetylspermidine oxidase (propane-1,3-diamine-forming) (EC 1.5.3.15) is an enzyme with systematic name N8-acetylspermidine:oxygen oxidoreductase (propane-1,3-diamine-forming). This enzyme catalyses the following chemical reaction
Spermine oxidase (EC 1.5.3.16, PAOh1/SMO, AtPAO1, AtPAO4, SMO) is an enzyme with systematic name spermidine:oxygen oxidoreductase (spermidine-forming). This enzyme catalyses the following chemical reaction
Non-specific polyamine oxidase (EC 1.5.3.17, polyamine oxidase, Fms1, AtPAO3) is an enzyme with systematic name polyamine:oxygen oxidoreductase (3-aminopropanal or 3-acetamidopropanal-forming). This enzyme catalyses the following chemical reaction
Homospermidine synthase (EC 2.5.1.44) is an enzyme with systematic name putrescine:putrescine 4-aminobutyltransferase (ammonia-forming). This enzyme catalyses the following chemical reaction
Deoxyhypusine synthase (EC 2.5.1.46, spermidine:eIF5A-lysine 4-aminobutyltransferase (propane-1,3-diamine-forming)) is an enzyme with systematic name (eIF5A-precursor)-lysine:spermidine 4-aminobutyltransferase (propane-1,3-diamine-forming). This enzyme catalyses the following chemical reaction






