Pseudomonas denitrificans is a Gram-negative aerobic bacterium that performs denitrification. It was first isolated from garden soil in Vienna, Austria. It overproduces cobalamin (vitamin B12), which it uses for methionine synthesis and it has been used for manufacture of the vitamin. Scientists at Rhône-Poulenc Rorer took a genetically engineered strain of the bacteria, in which eight of the cob genes involved in the biosynthesis of the vitamin had been overexpressed, to establish the complete sequence of methylation and other steps in the cobalamin pathway.
In enzymology, a cobalt-factor II C20-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a magnesium protoporphyrin IX methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-2 C20-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, precorrin-3B C17-methyltransferase is an enzyme that catalyzes the chemical reaction
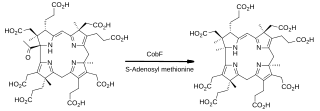
In enzymology, precorrin-6A synthase (deacetylating) (EC 2.1.1.152) is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-6Y C5,15-methyltransferase (decarboxylating) (EC 2.1.1.132) is an enzyme that catalyzes the chemical reaction
In enzymology, a rRNA (adenine-N6-)-methyltransferase (EC 2.1.1.48) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-6A reductase (EC 1.3.1.54) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-3B synthase (EC 1.14.13.83) is an enzyme that catalyzes the chemical reaction

[Methionine synthase] reductase, or Methionine synthase reductase, encoded by the gene MTRR, is an enzyme that is responsible for the reduction of methionine synthase inside human body. This enzyme is crucial for maintaining the one carbon metabolism, specifically the folate cycle. The enzyme employs one coenzyme, flavoprotein.

In enzymology, a precorrin-8X methylmutase is an enzyme that catalyzes the chemical reaction

Cobalt chelatase (EC 6.6.1.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

In molecular biology, cob(I)yrinic acid a,c-diamide adenosyltransferase EC 2.5.1.17 is an enzyme which catalyses the conversion of cobalamin into one of its coenzyme forms, adenosylcobalamin. Adenosylcobalamin is required as a cofactor for the activity of certain enzymes. AdoCbl contains an adenosyl moiety liganded to the cobalt ion of cobalamin via a covalent Co-C bond.

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.

Uroporphyrinogen-III C-methyltransferase, uroporphyrinogen methyltransferase, uroporphyrinogen-III methyltransferase, adenosylmethionine-uroporphyrinogen III methyltransferase, S-adenosyl-L-methionine-dependent uroporphyrinogen III methylase, uroporphyrinogen-III methylase, SirA, CysG, CobA, uroporphyrin-III C-methyltransferase, S-adenosyl-L-methionine:uroporphyrin-III C-methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:uroporphyrinogen-III C-methyltransferase. This enzyme catalyses the following chemical reaction
Cobalt-precorrin-5B (C1)-methyltransferase (EC 2.1.1.195), cobalt-precorrin-6A synthase, CbiD (gene)) is an enzyme with systematic name S-adenosyl-L-methionine:cobalt-precorrin-5B (C1)-methyltransferase. This enzyme catalyses the following chemical reaction
Cobalt-precorrin-7 (C15)-methyltransferase (decarboxylating) (EC 2.1.1.196, CbiT) is an enzyme with systematic name S-adenosyl-L-methionine:precorrin-7 C15-methyltransferase (C12-decarboxylating). This enzyme catalyses the following chemical reaction
Erythromycin 3''-O-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:erythromycin C 3''-O-methyltransferase. This enzyme catalyses the following chemical reaction












