Related Research Articles

Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.

Anaplastic large-cell lymphoma (ALCL) refers to a group of non-Hodgkin lymphomas in which aberrant T cells proliferate uncontrollably. Considered as a single entity, ALCL is the most common type of peripheral lymphoma and represents ~10% of all peripheral lymphomas in children. The incidence of ALCL is estimated to be 0.25 cases per 100,000 people in the United States of America. There are four distinct types of anaplastic large-cell lymphomas that on microscopic examination share certain key histopathological features and tumor marker proteins. However, the four types have very different clinical presentations, gene abnormalities, prognoses, and/or treatments.
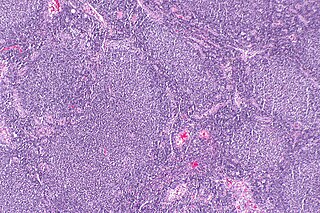
Follicular lymphoma (FL) is a cancer that involves certain types of white blood cells known as lymphocytes. The cancer originates from the uncontrolled division of specific types of B-cells known as centrocytes and centroblasts. These cells normally occupy the follicles (nodular swirls of various types of lymphocytes) in the germinal centers of lymphoid tissues such as lymph nodes. The cancerous cells in FL typically form follicular or follicle-like structures (see adjacent Figure) in the tissues they invade. These structures are usually the dominant histological feature of this cancer.
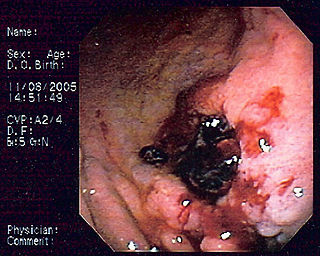
MALT lymphoma is a form of lymphoma involving the mucosa-associated lymphoid tissue (MALT), frequently of the stomach, but virtually any mucosal site can be affected. It is a cancer originating from B cells in the marginal zone of the MALT, and is also called extranodal marginal zone B cell lymphoma.
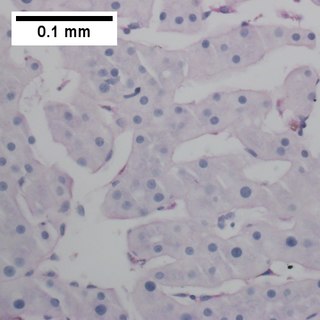
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.
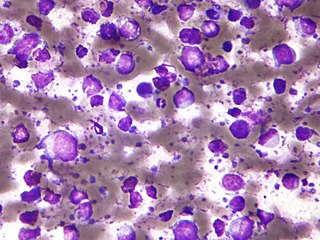
The B-cell lymphomas are types of lymphoma affecting B cells. Lymphomas are "blood cancers" in the lymph nodes. They develop more frequently in older adults and in immunocompromised individuals.

Intravascular lymphomas (IVL) are rare cancers in which malignant lymphocytes proliferate and accumulate within blood vessels. Almost all other types of lymphoma involve the proliferation and accumulation of malignant lymphocytes in lymph nodes, other parts of the lymphatic system, and various non-lymphatic organs but not in blood vessels.
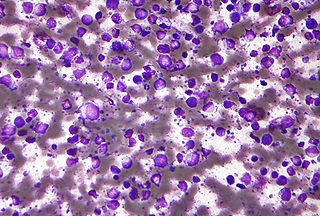
Diffuse large B-cell lymphoma (DLBCL) is a cancer of B cells, a type of lymphocyte that is responsible for producing antibodies. It is the most common form of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year in the US and UK. This cancer occurs primarily in older individuals, with a median age of diagnosis at ~70 years, although it can occur in young adults and, in rare cases, children. DLBCL can arise in virtually any part of the body and, depending on various factors, is often a very aggressive malignancy. The first sign of this illness is typically the observation of a rapidly growing mass or tissue infiltration that is sometimes associated with systemic B symptoms, e.g. fever, weight loss, and night sweats.
Richter's transformation (RT), also known as Richter's syndrome, is the conversion of chronic lymphocytic leukemia (CLL) or its variant, small lymphocytic lymphoma (SLL), into a new and more aggressively malignant disease. CLL is the circulation of malignant B lymphocytes with or without the infiltration of these cells into lymphatic or other tissues while SLL is the infiltration of these malignant B lymphocytes into lymphatic and/or other tissues with little or no circulation of these cells in the blood. CLL along with its SLL variant are grouped together in the term CLL/SLL.

Marginal zone B-cell lymphomas, also known as marginal zone lymphomas (MZLs), are a heterogeneous group of lymphomas that derive from the malignant transformation of marginal zone B-cells. Marginal zone B cells are innate lymphoid cells that normally function by rapidly mounting IgM antibody immune responses to antigens such as those presented by infectious agents and damaged tissues. They are lymphocytes of the B-cell line that originate and mature in secondary lymphoid follicles and then move to the marginal zones of mucosa-associated lymphoid tissue, the spleen, or lymph nodes. Mucosa-associated lymphoid tissue is a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body such as the gastrointestinal tract, mouth, nasal cavity, pharynx, thyroid gland, breast, lung, salivary glands, eye, skin and the human spleen.

Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is a slow-growing CD20 positive form of Hodgkin lymphoma, a cancer of the immune system's B cells.
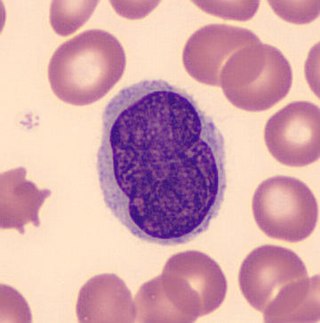
Lutzner cells were discovered by Marvin A. Lutzner, Lucien-Marie Pautrier, and Albert Sézary. These cells are described as the smaller forms of Sézary cells, or Sézary-Lutzner cells, and the two variants are recognised as being morphologically different. Aggregates of these cells in mycosis fungoides are known as a Pautrier's microabscesses. They are a form of T-lymphocytes that has been mutated This atypical form of T-lymphocytes contains T-cell receptors on the surface and is found in both the dermis and epidermis layers of the skin. Since Lutzner cells are a mutated form of T-lymphocytes, they develop in bone marrow and are transported to the thymus is order to mature. The production and maturation stages occur before the cell has developed a mutation. Lutzner cells can form cutaneous T-cell lymphoma, which is a form of skin cancer.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.

Plasmablastic lymphoma (PBL) is a type of large B-cell lymphoma recognized by the World Health Organization (WHO) in 2017 as belonging to a subgroup of lymphomas termed lymphoid neoplasms with plasmablastic differentiation. The other lymphoid neoplasms within this subgroup are: plasmablastic plasma cell lymphoma ; primary effusion lymphoma that is Kaposi's sarcoma-associated herpesvirus positive or Kaposi's sarcoma-associated Herpesvirus negative; anaplastic lymphoma kinase-positive large B-cell lymphoma; and human herpesvirus 8-positive diffuse large B-cell lymphoma, not otherwise specified. All of these lymphomas are malignancies of plasmablasts, i.e. B-cells that have differentiated into plasmablasts but because of their malignant nature: fail to differentiate further into mature plasma cells; proliferate excessively; and accumulate in and injure various tissues and organs.
Epstein–Barr virus–associated lymphoproliferative diseases are a group of disorders in which one or more types of lymphoid cells, i.e. B cells, T cells, NK cells, and histiocytic-dendritic cells, are infected with the Epstein–Barr virus (EBV). This causes the infected cells to divide excessively, and is associated with the development of various non-cancerous, pre-cancerous, and cancerous lymphoproliferative disorders (LPDs). These LPDs include the well-known disorder occurring during the initial infection with the EBV, infectious mononucleosis, and the large number of subsequent disorders that may occur thereafter. The virus is usually involved in the development and/or progression of these LPDs although in some cases it may be an "innocent" bystander, i.e. present in, but not contributing to, the disease.
Duodenal-type follicular lymphoma (DFL) is a form of lymphoma in which certain lymphocyte types, the B-cell-derived centrocytes and centroblasts, form lymph node follicle-like structures principally in the duodenum and other parts of the small intestine. It is an indolent disease which on rare occasions progresses to a more aggressive lymphoma that spreads beyond these originally involved sites.
T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is a malignancy of B cells. B-cells are lymphocytes that normally function in the humoral immunity component of the adaptive immune system by secreting antibodies that, for example, bind to and neutralize invasive pathogens. Among the various forms of B-cell lymphomas, THRLBCL is a rarely occurring subtype of the diffuse large B-cell lymphomas (DLBCL). DLBCL are a large group of lymphomas that account for ~25% of all non-Hodgkin lymphomas worldwide. THRLBCL is distinguished from the other DLBCL subtypes by the predominance of non-malignant T-cell lymphocytes and histiocytes over malignant B-cells in its tumors and tissue infiltrates.
Primary testicular diffuse large B-cell lymphoma (PT-DLBCL), also termed testicular diffuse large B-cell lymphoma and diffuse large B-cell lymphoma of the testes, is a variant of the diffuse large B-cell lymphomas (DLBCL). DLBCL are a large and diverse group of B-cell malignancies with the great majority (-85%) being typed as diffuse large B-cell lymphoma, not otherwise specified. PT-DLBCL is a variant of DLBCL, NOS that involves one or, in uncommon cases, both testicles. Other variants and subtypes of DLBCL may involve the testes by spreading to them from their primary sites of origin in other tissues. PT-DLBCL differs from these other DLBCL in that it begins in the testes and then may spread to other sites.
Diffuse large B-cell lymphoma associated with chronic inflammation (DLBCL-CI) is a subtype of the Diffuse large B-cell lymphomas and a rare form of the Epstein–Barr virus-associated lymphoproliferative diseases, i.e. conditions in which lymphocytes infected with the Epstein-Barr virus (EBV) proliferate excessively in one or more tissues. EBV infects ~95% of the world's population to cause no symptoms, minor non-specific symptoms, or infectious mononucleosis. The virus then enters a latency phase in which the infected individual becomes a lifetime asymptomatic carrier of the virus. Some weeks, months, years, or decades thereafter, a very small fraction of these carriers, particularly those with an immunodeficiency, develop any one of various EBV-associated benign or malignant diseases.
Fibrin-associated diffuse large B-cell lymphoma (FA-DLBCL) is an extremely rare form of the diffuse large B-cell lymphomas (DLBCL). DLBCL are lymphomas in which a particular type of lymphocyte, the B-cell, proliferates excessively, invades multiple tissues, and often causes life-threatening tissue damage. DLBCL have various forms as exemplified by one of its subtypes, diffuse large B-cell lymphoma associated with chronic inflammation (DLBCL-CI). DLBCL-CI is an aggressive malignancy that develops in sites of chronic inflammation that are walled off from the immune system. In this protected environment, the B-cells proliferate excessively, acquire malignant gene changes, form tumor masses, and often spread outside of the protected environment. In 2016, the World Health Organization provisionally classified FA-DLBCL as a DLBCL-CI. Similar to DLBCL-CI, FA-DLBCL involves the proliferation of EBV-infected large B-cells in restricted anatomical spaces that afford protection from an individual's immune system. However, FA-DLBCL differs from DLBCL-CI in many other ways, including, most importantly, its comparatively benign nature. Some researchers have suggested that this disease should be regarded as a non-malignant or pre-malignant lymphoproliferative disorder rather than a malignant DLBCL-CI.
References
- 1 2 3 4 5 6 Chen ST, Barnes J, Duncan L (March 2018). "Primary cutaneous B-cell lymphomas- clinical and histopathologic features, differential diagnosis, and treatment". Seminars in Cutaneous Medicine and Surgery. 37 (1): 49–55. doi:10.12788/j.sder.2018.014. PMID 29719020. S2CID 22888927.
- ↑ Jia J, Li W, Zheng Y (February 2017). "Primary cutaneous diffuse large B cell lymphoma-other successfully treated by the combination of R-CHOP chemotherapy and surgery: A case report and review of literature". Medicine. 96 (8): e6161. doi:10.1097/MD.0000000000006161. PMC 5569421 . PMID 28225499.
- 1 2 3 4 5 6 7 8 Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ (November 2019). "Diffuse large B-cell lymphoma variants: an update". Pathology. 52 (1): 53–67. doi:10.1016/j.pathol.2019.08.013. PMID 31735345.
- 1 2 3 4 Grimm KE, O'Malley DP (February 2019). "Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues". Annals of Diagnostic Pathology. 38: 6–10. doi:10.1016/j.anndiagpath.2018.09.014. PMID 30380402. S2CID 53196244.
- 1 2 3 4 5 6 7 8 9 10 11 12 Selva R, Violetti SA, Delfino C, Grandi V, Cicchelli S, Tomasini C, Fierro MT, Berti E, Pimpinelli N, Quaglino P (2017). "A Literature Revision in Primary Cutaneous B-cell Lymphoma". Indian Journal of Dermatology. 62 (2): 146–157. doi: 10.4103/ijd.IJD_74_17 . PMC 5363138 . PMID 28400634.
- 1 2 3 4 Jaffe ES (January 2020). "Navigating the cutaneous B-cell lymphomas: avoiding the rocky shoals". Modern Pathology. 33 (Suppl 1): 96–106. doi: 10.1038/s41379-019-0385-7 . PMID 31653979. S2CID 204887118.
- 1 2 3 4 5 6 7 8 9 10 11 Tadiotto Cicogna G, Ferranti M, Lazzarotto A, Alaibac M (2019). "Biological Approaches to Aggressive Cutaneous B-Cell Lymphomas". Frontiers in Oncology. 9: 1238. doi: 10.3389/fonc.2019.01238 . PMC 6864397 . PMID 31799195.
- 1 2 3 Wilcox RA (November 2018). "Cutaneous B-cell lymphomas: 2019 update on diagnosis, risk stratification, and management". American Journal of Hematology. 93 (11): 1427–1430. doi: 10.1002/ajh.25224 . PMID 30039522.
- ↑ Cabanillas F, Shah B (December 2017). "Advances in Diagnosis and Management of Diffuse Large B-cell Lymphoma". Clinical Lymphoma, Myeloma & Leukemia. 17 (12): 783–796. doi:10.1016/j.clml.2017.10.007. PMID 29126866. S2CID 25304758.
- ↑ Chavez JC, Locke FL (June 2018). "CAR T cell therapy for B-cell lymphomas". Best Practice & Research. Clinical Haematology. 31 (2): 135–146. doi:10.1016/j.beha.2018.04.001. PMC 6716161 . PMID 29909914.
- ↑ Gravelle P, Burroni B, Péricart S, Rossi C, Bezombes C, Tosolini M, Damotte D, Brousset P, Fournié JJ, Laurent C (July 2017). "Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies". Oncotarget. 8 (27): 44960–44975. doi:10.18632/oncotarget.16680. PMC 5546533 . PMID 28402953.
- ↑ Heyman M, Einhorn S (October 1996). "Inactivation of the p15INK4B and p16INK4 genes in hematologic malignancies". Leukemia & Lymphoma. 23 (3–4): 235–45. doi:10.3109/10428199609054826. PMID 9031104.
- ↑ Abbas O, Mahalingam M (February 2013). "The grenz zone". The American Journal of Dermatopathology. 35 (1): 83–91. doi:10.1097/DAD.0b013e31824feb4e. PMID 23348142. S2CID 24048289.
- ↑ Ollila TA, Olszewski AJ (June 2018). "Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence". Current Treatment Options in Oncology. 19 (8): 38. doi:10.1007/s11864-018-0555-8. PMC 6294323 . PMID 29931605.
- ↑ Li S, Young KH, Medeiros LJ (January 2018). "Diffuse large B-cell lymphoma". Pathology. 50 (1): 74–87. doi:10.1016/j.pathol.2017.09.006. PMID 29167021. S2CID 20839613.[ permanent dead link ]
- ↑ "International Society for Cutaneous Lymphomas (ISCL) > About the ISCL". www.cutaneouslymphoma.org. Archived from the original on 2015-05-09.
- ↑ "A Randomized Phase 2 Study of CDX-1127 (Varlilumab) in Combination with Nivolumab in Patients with Relapsed or Refractory Aggressive B-cell Lymphomas". 23 October 2021.