
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin itself contains no molybdenum; rather, this is the name of the ligand that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor.
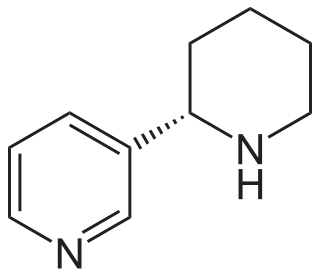
Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco plant, as well as in the close relative of the common tobacco plant. It is a structural isomer of, and chemically similar to, nicotine. Its principal (historical) industrial use is as an insecticide.

Monoamine oxidase B, also known as MAOB, is an enzyme that in humans is encoded by the MAOB gene.
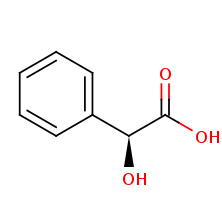
In enzymology, (S)-mandelate dehydrogenase (MDH), is an enzyme that catalyzes the chemical reaction.
In enzymology, an indole-3-acetaldehyde oxidase (EC 1.2.3.7) is an enzyme that catalyzes the chemical reaction
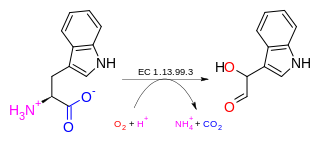
In enzymology, a tryptophan 2'-dioxygenase is an enzyme that catalyzes the chemical reaction

In enzymology, an L-amino acid oxidase (LAAO) (EC 1.4.3.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-methyl-L-amino-acid oxidase (EC 1.5.3.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-6-hydroxynicotine oxidase (EC 1.5.3.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-6-hydroxynicotine oxidase (EC 1.5.3.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a mimosinase (EC 3.5.1.61) is an enzyme that catalyzes the chemical reaction
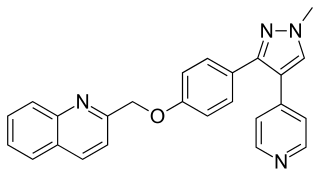
Mardepodect is a drug which was developed by Pfizer for the treatment of schizophrenia. It acts as a phosphodiesterase inhibitor selective for the PDE10A subtype. The PDE10A enzyme is expressed primarily in the brain, mostly in the striatum, nucleus accumbens and olfactory tubercle, and is thought to be particularly important in regulating the activity of dopamine-sensitive medium spiny neurons in the striatum which are known to be targets of conventional antipsychotic drugs. Older PDE10A inhibitors such as papaverine have been shown to produce antipsychotic effects in animal models, and more potent and selective PDE10A inhibitors are a current area of research for novel antipsychotic drugs which act through a different pathway to conventional dopamine or 5-HT2A antagonist drugs and may have a more favourable side effects profile. Mardepodect is currently one of the furthest advanced PDE10A inhibitors in development and has progressed through to Phase II clinical trials in humans. In 2017, development of mardepodect for the treatment of schizophrenia and Huntington's disease was discontinued.

TC-1698 is a drug developed by Targacept which acts as a partial agonist for the α7 subtype of neural nicotinic acetylcholine receptors. It has neuroprotective effects in animal studies, and has been used as a lead compound to find further potent derivatives.

Almoxatone (MD-780,236) is a selective and reversible inhibitor of MAO-B. It was patented as an antidepressant and antiparkinsonian agent but was never marketed.
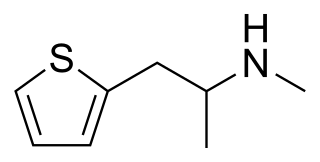
Methiopropamine (MPA) is an organic compound structurally related to methamphetamine. Originally reported in 1942, the molecule consists of a thiophene group with an alkyl amine substituent at the 2-position. It appeared for public sale in the UK in December 2010 as a "research chemical" or "legal high", recently branded as Blow. It has limited popularity as a recreational stimulant.
3-Succinoylsemialdehyde-pyridine dehydrogenase (EC 1.2.1.83) is an enzyme with systematic name 4-oxo-4-(pyridin-3-yl)butanal:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
7-Chloro-L-tryptophan oxidase (EC 1.4.3.23, RebO) is an enzyme with systematic name 7-chloro-L-tryptophan:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction
4-methylaminobutanoate oxidase (methylamine-forming) (EC 1.5.3.21, mao (gene)) is an enzyme with systematic name 4-methylaminobutanoate methylamidohydrolase. This enzyme catalyses the following chemical reaction
6-hydroxypseudooxynicotine dehydrogenase (EC 1.5.99.14) is an enzyme with systematic name 1-(6-hydroxypyridin-3-yl)-4-(methylamino)butan-1-one:acceptor 6-oxidoreductase (hydroxylating). This enzyme catalyses the following chemical reaction:
2,6-dihydroxypseudooxynicotine hydrolase (EC 3.7.1.19) is an enzyme with systematic name 1-(2,6-dihydroxypyridin-3-yl)-4-(methylamino)butan-1-one hydrolase. This enzyme catalyses the following chemical reaction










