Related Research Articles
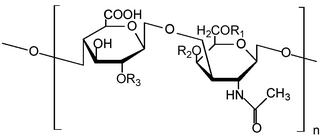
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.
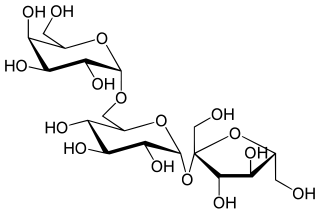
Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme which in the lumen of the human digestive tract is only produced by bacteria in the large intestine. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. The enzyme does not cleave β-linked galactose, as in lactose.
Isomaltase is an enzyme that breaks the bonds linking saccharides, which cannot be broken by amylase or maltase. It digests polysaccharides at the alpha 1-6 linkages. Its substrate, alpha-limit dextrin, is a product of amylopectin digestion that retains its 1-6 linkage. The product of the enzymatic digestion of alpha-limit dextrin by isomaltase is maltose.

α-Galactosidase is a glycoside hydrolase enzyme that catalyses the following reaction:

Sialyltransferases are enzymes that transfer sialic acid to nascent oligosaccharide. Each sialyltransferase is specific for a particular sugar substrate. Sialyltransferases add sialic acid to the terminal portions of the sialylated glycolipids (gangliosides) or to the N- or O-linked sugar chains of glycoproteins.
In enzymology, a polysaccharide O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a galactosyldiacylglycerol alpha-2,3-sialyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetyllactosaminide 3-alpha-galactosyltransferase is an enzyme that catalyzes the chemical reaction

Polypeptide N-acetylgalactosaminyltransferase 2 is an enzyme that in humans is encoded by the GALNT2 gene.
Beta-D-galactosyl-(1->4)-L-rhamnose phosphorylase is an enzyme with systematic name beta-D-galactosyl-(1->4)-L-rhamnose:phosphate 1-alpha-D-galactosyltransferase. This enzyme catalyses the following chemical reaction
Dolichyl-P-Man:Man5GlcNAc2-PP-dolichol alpha-1,3-mannosyltransferase is an enzyme with systematic name dolichyl beta-D-mannosyl phosphate:D-Man-alpha-(1->2)-D-Man-alpha-(1->2)-D-Man-alpha-(1->3)-(D-Man-alpha- )-D-Man-beta-(1->4)-D-GlcNAc-beta-(1->4)-D-GlcNAc-diphosphodolichol alpha-1,3-mannosyltransferase. This enzyme catalyses the following chemical reaction
Endo-α-N-acetylgalactosaminidase (EC 3.2.1.97, endo-α-acetylgalactosaminidase, endo-α-N-acetyl-D-galactosaminidase, mucinaminylserine mucinaminidase, D-galactosyl-3-(N-acetyl-α-D-galactosaminyl)-L-serine mucinaminohydrolase, endo-α-GalNAc-ase, D-galactosyl-N-acetyl-α-D-galactosamine D-galactosyl-N-acetyl-galactosaminohydrolase) is an enzyme with systematic name glycopeptide-D-galactosyl-N-acetyl-α-D-galactosamine D-galactosyl-N-acetyl-galactosaminohydrolase. This enzyme catalyses the following chemical reaction
Lacto-N-biosidase (EC 3.2.1.140) is an enzyme with systematic name oligosaccharide lacto-N-biosylhydrolase. This enzyme catalyses the following chemical reaction
Alpha-neoagaro-oligosaccharide hydrolase is an enzyme with systematic name alpha-neoagaro-oligosaccharide 3-glycohydrolase. This enzyme catalyses the following chemical reaction
Rhamnogalacturonan hydrolase is an enzyme with systematic name rhamnogalacturonan alpha-D-GalA-(1->2)-alpha-L-Rha hydrolase. This enzyme catalyses the following chemical reaction
Rhamnogalacturonan rhamnohydrolase is an enzyme with systematic name rhamnogalacturonan oligosaccharide alpha-L-Rha-(1->4)-alpha-D-GalA rhamnohydrolase. This enzyme catalyses the following chemical reaction
Alpha-D-xyloside xylohydrolase is an enzyme. This enzyme catalyses the following chemical reaction
Rhamnogalacturonan exolyase is an enzyme with systematic name α-L-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronate exolyase. This enzyme catalyses the following chemical reaction
The enzyme Rhamnogalacturonan endolyase is an enzyme with systematic name α-L-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronate endolyase. catalyses the following process:
Human milk oligosaccharides (HMOs), also known as human milk glycans, are short polymers of simple sugars that can be found in high concentrations in human breast milk. Human milk oligosaccharides promote the development of the immune system, can reduce the risk of pathogen infections and improve brain development and cognition. The HMO profile of human breast milk shapes the gut microbiota of the infant by selectively stimulating bifidobacteria and other bacteria.
References
- ↑ Mutter M, Beldman G, Pitson SM, Schols HA, Voragen AG (May 1998). "Rhamnogalacturonan alpha-d-galactopyranosyluronohydrolase. An enzyme that specifically removes the terminal nonreducing galacturonosyl residue in rhamnogalacturonan regions of pectin". Plant Physiology. 117 (1): 153–63. doi:10.1104/pp.117.1.153. PMC 34998 . PMID 9576784.