
An exoenzyme, or extracellular enzyme, is an enzyme that is secreted by a cell and functions outside that cell. Exoenzymes are produced by both prokaryotic and eukaryotic cells and have been shown to be a crucial component of many biological processes. Most often these enzymes are involved in the breakdown of larger macromolecules. The breakdown of these larger macromolecules is critical for allowing their constituents to pass through the cell membrane and enter into the cell. For humans and other complex organisms, this process is best characterized by the digestive system which breaks down solid food via exoenzymes. The small molecules, generated by the exoenzyme activity, enter into cells and are utilized for various cellular functions. Bacteria and fungi also produce exoenzymes to digest nutrients in their environment, and these organisms can be used to conduct laboratory assays to identify the presence and function of such exoenzymes. Some pathogenic species also use exoenzymes as virulence factors to assist in the spread of these disease-causing microorganisms. In addition to the integral roles in biological systems, different classes of microbial exoenzymes have been used by humans since pre-historic times for such diverse purposes as food production, biofuels, textile production and in the paper industry. Another important role that microbial exoenzymes serve is in the natural ecology and bioremediation of terrestrial and marine environments.

Trehalose is a sugar consisting of two molecules of glucose. It is also known as mycose or tremalose. Some bacteria, fungi, plants and invertebrate animals synthesize it as a source of energy, and to survive freezing and lack of water.

Rhizopus is a genus of common saprophytic fungi on plants and specialized parasites on animals. They are found in a wide variety of organic substances, including "mature fruits and vegetables", jellies, syrups, leather, bread, peanuts, and tobacco. They are multicellular. Some Rhizopus species are opportunistic human pathogens that often cause fatal disease called mucormycosis. This widespread genus includes at least eight species.

α-Glucosidase (EC 3.2.1.20, is a glucosidase located in the brush border of the small intestine that acts upon α bonds:
Rhizopus arrhizus is a fungus of the family Mucoraceae, characterized by sporangiophores that arise from nodes at the point where the rhizoids are formed and by a hemispherical columella. It is the most common cause of mucormycosis in humans and occasionally infects other animals.

Sucrase-isomaltase is a bifunctional glucosidase located on the brush border of the small intestine, encoded by the human gene SI. It is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. It has preferential expression in the apical membranes of enterocytes. The enzyme’s purpose is to digest dietary carbohydrates such as starch, sucrose and isomaltose. By further processing the broken-down products, energy in the form of ATP can be generated.

Eosinophil-derived neurotoxin is an enzyme that in humans is encoded by the RNASE2 gene.

Sake kasu (酒粕) or sake lees is the name given to the pressed lees left over from the production of sake. It is used as a cooking ingredient that is white in color, having a paste-like texture. The taste is fruity and similar to sake itself. A by-product of Japanese sake production, it typically contains 8% alcohol, has high nutritional value, and might have health benefits.
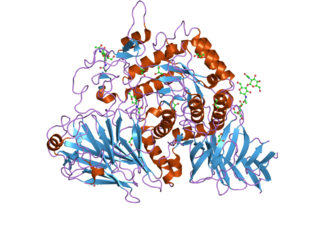
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the MGAM gene.

α-Glucans (alpha-glucans) are polysaccharides of D-glucose monomers linked with glycosidic bonds of the alpha form. α-Glucans use cofactors in a cofactor site in order to activate a glucan phosphorylase enzyme. This enzyme causes a reaction that transfers a glucosyl portion between orthophosphate and α-I,4-glucan. The position of the cofactors to the active sites on the enzyme are critical to the overall reaction rate thus, any alteration to the cofactor site leads to the disruption of the glucan binding site.
Rhizomucor miehei is a species of fungus. It is commercially used to produce enzymes which can be used to produce a microbial rennet to curd milk and produce cheese.
In the food industry and biochemistry, interesterification (IE) is a process that rearranges the fatty acids of a fat product, typically a mixture of triglycerides. The process implies breaking and reforming the ester bonds C–O–C that connect the fatty acid chains to the glycerol hubs of the fat molecules. The reactions involve catalysts, either inorganic chemicals or enzymes.

Jiuqu, also simply known as qu is a type of dried fermentation starter used in the production of traditional Chinese alcoholic beverages. The word jiuqu specifically refers to a type of yeast used to make alcohol such as huangjiu, baijiu and jiuniang.
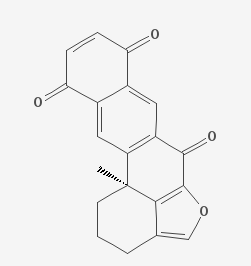
Xestoquinone is a bio-active isolate of the marine sponge Xestospongia.
Moesziomyces antarcticus is a species of fungus in the order Ustilaginales. The species occurs as a yeast and was originally isolated from Antarctic lake sediment. It is a rare cause of human fungaemia infections.
Rhodococcus erythropolis is a bacterium species in the genus Rhodococcus. It is Gram-positive. R. erythropolis has been isolated from the air of the Russian Space Laboratory Mir along with a large number of other microorganisms that steadily accumulated during the lifespan of the station. Rhodococcus bacteria are known to degrade organic compounds contained in the rubber used aboard the space station with specialized enzymes. This can lead to degradation of critical components and necessitates replacement of the parts or preventive measures dealing with microbial contamination.

Rhizopus oryzae is a filamentous heterothallic microfungus that occurs as a saprotroph in soil, dung, and rotting vegetation. This species is very similar to Rhizopus stolonifer, but it can be distinguished by its smaller sporangia and air-dispersed sporangiospores. It differs from R. oligosporus and R. microsporus by its larger columellae and sporangiospores. The many strains of R. oryzae produce a wide range of enzymes such as carbohydrate digesting enzymes and polymers along with a number of organic acids, ethanol and esters giving it useful properties within the food industries, bio-diesel production, and pharmaceutical industries. It is also an opportunistic pathogen of humans causing mucormycosis.
Thermomyces lanuginosus is a species of thermophilic fungus that belongs to Thermomyces, a genus of hemicellulose degraders. It is classified as a deuteromycete and no sexual form has ever been observed. It is the dominant fungus of compost heaps, due to its ability to withstand high temperatures and use complex carbon sources for energy. As the temperature of compost heaps rises and the availability of simple carbon sources decreases, it is able to out compete pioneer microflora. It plays an important role in breaking down the hemicelluloses found in plant biomass due to the many hydrolytic enzymes that it produces, such as lipolase, amylase, xylanase, phytase, and chitinase. These enzymes have chemical, environmental, and industrial applications due to their hydrolytic properties. They are used in the food, petroleum, pulp and paper, and animal feed industries, among others. A few rare cases of endocarditis due to T. lanuginosus have been reported in humans.

Monitor peptide, also known as pancreatic secretory trypsin inhibitor I (PSTI-I) or pancreatic secretory trypsin inhibitor 61 (PSTI-61), is a peptide that plays an important role in the regulation of the digestive system, specifically the release of cholecystokinin (CCK).

Butyl oleate is a fatty acid ester and an organic chemical found in liquid form. It has the formula C22H42O2 and the CAS Registry Number 142-77-8. It is REACH registered and produced or imported into the European Union with the EC number of 205-559-6.













