
Cubic zirconia (abbreviated CZ) is the cubic crystalline form of zirconium dioxide (ZrO2). The synthesized material is hard and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate (ZrSiO4). It is sometimes erroneously called cubic zirconium.

Zone melting is a group of similar methods of purifying crystals, in which a narrow region of a crystal is melted, and this molten zone is moved along the crystal. The molten region melts impure solid at its forward edge and leaves a wake of purer material solidified behind it as it moves through the ingot. The impurities concentrate in the melt, and are moved to one end of the ingot. Zone refining was invented by John Desmond Bernal and further developed by William G. Pfann in Bell Labs as a method to prepare high-purity materials, mainly semiconductors, for manufacturing transistors. Its first commercial use was in germanium, refined to one atom of impurity per ten billion, but the process can be extended to virtually any solute–solvent system having an appreciable concentration difference between solid and liquid phases at equilibrium. This process is also known as the float zone process, particularly in semiconductor materials processing.

The Czochralski method, also Czochralski technique or Czochralski process, is a method of crystal growth used to obtain single crystals of semiconductors, metals, salts and synthetic gemstones. The method is named after Polish scientist Jan Czochralski, who invented the method in 1915 while investigating the crystallization rates of metals. He made this discovery by accident: instead of dipping his pen into his inkwell, he dipped it in molten tin, and drew a tin filament, which later proved to be a single crystal. The method is still used in over 90 percent of all electronics in the world that use semiconductors.

An ingot is a piece of relatively pure material, usually metal, that is cast into a shape suitable for further processing. In steelmaking, it is the first step among semi-finished casting products. Ingots usually require a second procedure of shaping, such as cold/hot working, cutting, or milling to produce a useful final product. Non-metallic and semiconductor materials prepared in bulk form may also be referred to as ingots, particularly when cast by mold based methods. Precious metal ingots can be used as currency, or as a currency reserve, as with gold bars.

Epitaxy refers to a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited crystalline film is called an epitaxial film or epitaxial layer. The relative orientation(s) of the epitaxial layer to the seed layer is defined in terms of the orientation of the crystal lattice of each material. For most epitaxial growths, the new layer is usually crystalline and each crystallographic domain of the overlayer must have a well-defined orientation relative to the substrate crystal structure. Epitaxy can involve single-crystal structures, although grain-to-grain epitaxy has been observed in granular films. For most technological applications, single-domain epitaxy, which is the growth of an overlayer crystal with one well-defined orientation with respect to the substrate crystal, is preferred. Epitaxy can also play an important role while growing superlattice structures.

The Bridgman–Stockbarger method, or Bridgman–Stockbarger technique, is named after physicist Percy Williams Bridgman (1882–1961) and physicist Donald C. Stockbarger (1895–1952). The method includes two similar but distinct techniques primarily used for growing boules, but which can be used for solidifying polycrystalline ingots as well.

In materials science, a single crystal is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries. The absence of the defects associated with grain boundaries can give monocrystals unique properties, particularly mechanical, optical and electrical, which can also be anisotropic, depending on the type of crystallographic structure. These properties, in addition to making some gems precious, are industrially used in technological applications, especially in optics and electronics.

Lanthanum gallium silicate (referred to as LGS in this article), also known as langasite, has a chemical formula of the form A3BC3D2O14, where A, B, C and D indicate particular cation sites. A is a decahedral (Thomson cube) site coordinated by 8 oxygen atoms. B is octahedral site coordinated by 6 oxygen atoms, and C and D are tetrahedral sites coordinated by 4 oxygen atoms. In this material, lanthanum occupied the A-sites, gallium the B, C and half of D-sites, and, silicon the other half of D-sites.
Synthetic alexandrite is an artificially grown crystalline variety of chrysoberyl, composed of beryllium aluminum oxide (BeAl2O4).
Chemical beam epitaxy (CBE) forms an important class of deposition techniques for semiconductor layer systems, especially III-V semiconductor systems. This form of epitaxial growth is performed in an ultrahigh vacuum system. The reactants are in the form of molecular beams of reactive gases, typically as the hydride or a metalorganic. The term CBE is often used interchangeably with metal-organic molecular beam epitaxy (MOMBE). The nomenclature does differentiate between the two processes, however. When used in the strictest sense, CBE refers to the technique in which both components are obtained from gaseous sources, while MOMBE refers to the technique in which the group III component is obtained from a gaseous source and the group V component from a solid source.

The Verneuil method, also called flame fusion, was the first commercially successful method of manufacturing synthetic gemstones, developed in the late 1883 by the French chemist Auguste Verneuil. It is primarily used to produce the ruby, sapphire and padparadscha varieties of corundum, as well as the diamond simulants rutile, strontium titanate and spinel. The principle of the process involves melting a finely powdered substance using an oxyhydrogen flame, and crystallising the melted droplets into a boule. The process is considered to be the founding step of modern industrial crystal growth technology, and remains in wide use to this day.
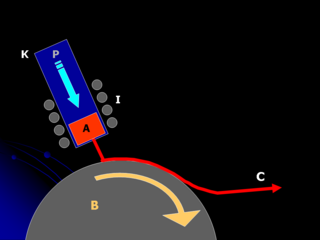
Melt spinning is a metal forming technique that is typically used to form thin ribbons of metal or alloys with a particular atomic structure.
Selective area epitaxy is the local growth of epitaxial layer through a patterned amorphous dielectric mask (typically SiO2 or Si3N4) deposited on a semiconductor substrate. Semiconductor growth conditions are selected to ensure epitaxial growth on the exposed substrate, but not on the dielectric mask. SAE can be executed in various epitaxial growth methods such as molecular beam epitaxy (MBE), metalorganic vapour phase epitaxy (MOVPE) and chemical beam epitaxy (CBE). By SAE, semiconductor nanostructures such as quantum dots and nanowires can be grown to their designed places.
Monocrystalline silicon, more often called single-crystal silicon, in short mono c-Si or mono-Si, is the base material for silicon-based discrete components and integrated circuits used in virtually all modern electronic equipment. Mono-Si also serves as a photovoltaic, light-absorbing material in the manufacture of solar cells.

Laser-heated pedestal growth (LHPG) or laser floating zone (LFZ) is a crystal growth technique. A narrow region of a crystal is melted with a powerful CO2 or YAG laser. The laser and hence the floating zone, is moved along the crystal. The molten region melts impure solid at its forward edge and leaves a wake of purer material solidified behind it. This technique for growing crystals from the melt is used in materials research.

The micro-pulling-down (μ-PD) method is a crystal growth technique based on continuous transport of the melted substance through micro-channel(s) made in a crucible bottom. Continuous solidification of the melt is progressed on a liquid/solid interface positioned under the crucible. In a steady state, both the melt and the crystal are pulled-down with a constant velocity.

Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV).
A rapidly increasing list of graphene production techniques have been developed to enable graphene's use in commercial applications.

The Kyropoulos method, also known as the KY method or Kyropoulos technique, is a method of bulk crystal growth used to obtain single crystals.
Two dimensional hexagonal boron nitride is a material of comparable structure to graphene with potential applications in e.g. photonics., fuel cells and as a substrate for two-dimensional heterostructures. 2D h-BN is isostructural to graphene, but where graphene is conductive, 2D h-BN is a wide-gap insulator.







