
Electrochemistry is the branch of physical chemistry that studies the relationship between electricity, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electricity considered an outcome of a particular chemical change or vice versa. These reactions involve electric charges moving between electrodes and an electrolyte. Thus electrochemistry deals with the interaction between electrical energy and chemical change.

An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic cells or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells. A common example of a galvanic cell is a standard 1.5 volt cell meant for consumer use. A battery consists of one or more cells, connected in parallel, series or series-and-parallel pattern.

A fuel cell is an electrochemical cell that converts the chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from metals and their ions or oxides that are commonly already present in the battery, except in flow batteries. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.

In chemistry and manufacturing, electrolysis is a technique that uses a direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential.
The Hall–Héroult process is the major industrial process for smelting aluminium. It involves dissolving aluminium oxide (alumina) in molten cryolite, and electrolysing the molten salt bath, typically in a purpose-built cell. The Hall–Héroult process applied at industrial scale happens at 940–980°C and produces 99.5–99.8% pure aluminium. Recycled aluminum requires no electrolysis, thus it does not end up in this process.
A regenerative fuel cell or reverse fuel cell (RFC) is a fuel cell run in reverse mode, which consumes electricity and chemical B to produce chemical A. By definition, the process of any fuel cell could be reversed. However, a given device is usually optimized for operating in one mode and may not be built in such a way that it can be operated backwards. Standard fuel cells operated backwards generally do not make very efficient systems unless they are purpose-built to do so as with high-pressure electrolysers, regenerative fuel cells, solid-oxide electrolyser cells and unitized regenerative fuel cells.

A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.
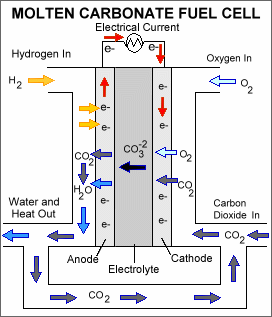
Molten-carbonate fuel cells (MCFCs) are high-temperature fuel cells that operate at temperatures of 600 °C and above.

The alkaline fuel cell (AFC), also known as the Bacon fuel cell) after its British inventor, Francis Thomas Bacon, is one of the most developed fuel cell technologies. NASA has used alkaline fuel cells since the mid-1960s, in Apollo-series missions and on the Space Shuttle.

High-temperature electrolysis is a technology for producing hydrogen from water at high temperatures.
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.
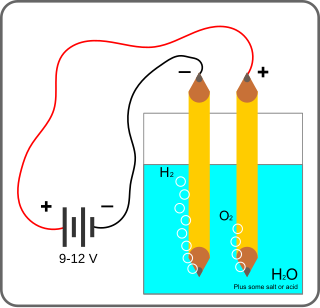
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to the passage of an electric current.
Aluminium smelting is the process of extracting aluminium from its oxide, alumina, generally by the Hall-Héroult process. Alumina is extracted from the ore bauxite by means of the Bayer process at an alumina refinery.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte. During discharging of a metal–air electrochemical cell, a reduction reaction occurs in the ambient air cathode while the metal anode is oxidized. The specific capacity and energy density of metal–air electrochemical cells is higher than that of lithium-ion batteries, making them a prime candidate for use in electric vehicles. However, complications associated with the metal anodes, catalysts, and electrolytes have hindered development and implementation of metal–air batteries.
Membraneless Fuel Cells convert stored chemical energy into electrical energy without the use of a conducting membrane as with other types of fuel cells. In Laminar Flow Fuel Cells (LFFC) this is achieved by exploiting the phenomenon of non-mixing laminar flows where the interface between the two flows works as a proton/ion conductor. The interface allows for high diffusivity and eliminates the need for costly membranes. The operating principles of these cells mean that they can only be built to millimeter-scale sizes. The lack of a membrane means they are cheaper but the size limits their use to portable applications which require small amounts of power.

An alkaline anion exchange membrane fuel cell (AAEMFC), also known as anion-exchange membrane fuel cells (AEMFCs), alkaline membrane fuel cells (AMFCs), hydroxide exchange membrane fuel cells (HEMFCs), or solid alkaline fuel cells (SAFCs) is a type of alkaline fuel cell that uses an anion exchange membrane to separate the anode and cathode compartments.

Proton exchange membrane (PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer.
Alkaline water electrolysis has a long history in the chemical industry. It is a type of electrolyzer that is characterized by having two electrodes operating in a liquid alkaline electrolyte solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH). These electrodes are separated by a diaphragm, separating the product gases and transporting the hydroxide ions (OH−) from one electrode to the other. A recent comparison showed that state-of-the-art nickel based water electrolyzers with alkaline electrolytes lead to competitive or even better efficiencies than acidic polymer electrolyte membrane water electrolysis with platinum group metal based electrocatalysts.

Mixed conductor which is known as mixed ion-electron conductor(MIEC) refers to a single-phase material which has a significant conduction ionically and electronically. Due to the mixed conduction, a formally neutral species can transport in a solid and therefore mass storage and redistribution are enabled. Mixed conductors are well known in conjugation with high-temperature superconductivity and are able to capacitate rapid solid-state reactions.





























