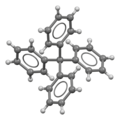
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,1′,1′′,1′′′-Methanetetrayltetrabenzene | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.132 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C25H20 | |||
| Molar mass | 320.44 g/mol | ||
| Melting point | 282 °C (540 °F; 555 K) | ||
| Boiling point | 431 °C | ||
| Structure [1] | |||
| tetragonal | |||
| P421c, No. 114 | |||
| S4 | |||
a = 10.896 Å, c = 7.280 Å (20 °C) | |||
Formula units (Z) | 2 | ||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Danger | |||
| H350 | |||
| P201, P202, P281, P308+P313, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
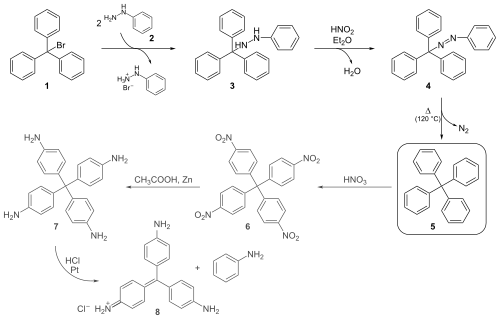
Tetraphenylmethane is an organic compound consisting of a methane core with four phenyl substituents. It was first synthesized by Moses Gomberg in 1898.