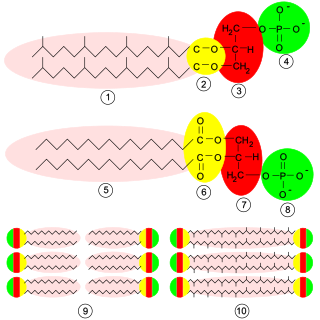
Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes. Two major classes are known: those for bacteria and eukaryotes and a separate family for archaea.

Lecithin–cholesterol acyltransferase is an enzyme, in many animals including humans, that converts free cholesterol into cholesteryl ester, which is then sequestered into the core of a lipoprotein particle, eventually making the newly synthesized HDL spherical and forcing the reaction to become unidirectional since the particles are removed from the surface. The enzyme is bound to high-density lipoproteins (HDLs) (alpha-LCAT) and LDLs (beta-LCAT) in the blood plasma. LCAT deficiency can cause impaired vision due to cholesterol corneal opacities, anemia, and kidney damage. It belongs to the family of phospholipid:diacylglycerol acyltransferases.
Diglyceride acyltransferase, DGAT, catalyzes the formation of triglycerides from diacylglycerol and fatty acyl-CoA. The reaction catalyzed by DGAT is considered the terminal and only committed step in triglyceride synthesis. The conversion is essential for intestinal absorption and adipose tissue formation.

Gastric lipase, also known as LIPF, is an enzymatic protein that, in humans, is encoded by the LIPF gene.
In enzymology, a 2-acylglycerol O-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-acylglycerol O-arachidonoyltransferase was an enzyme construed to catalyze the chemical reaction
In enzymology, a diacylglycerol-sterol O-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a long-chain-alcohol O-fatty-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphatidylcholine---sterol O-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phospholipid:diacylglycerol acyltransferase is an enzyme that catalyzes the chemical reaction
Sterol O-acyltransferase is an intracellular protein located in the endoplasmic reticulum that forms cholesteryl esters from cholesterol.

Sterol O-acyltransferase 1, also known as SOAT1, is an enzyme that in humans is encoded by the SOAT1 gene.

Sterol O-acyltransferase 2, also known as SOAT2, is an enzyme that in humans is encoded by the SOAT2 gene.

2-Acylglycerol O-acyltransferase 2 also known as acyl-CoA:monoacylglycerol acyltransferase 2 (MGAT2) or Diacylglycerol O-acyltransferase candidate 5 (DC5) is an enzyme that in humans is encoded by the MOGAT2 gene.

A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009.

The enzyme triacylglycerol lipase (also triglyceride lipase, EC 3.1.1.3;systematic name triacylglycerol acylhydrolase) catalyses the hydrolysis of ester linkages of triglycerides:

Diacylglycerol O-acyltransferase 1 is an enzyme that in humans is encoded by the DGAT1 gene.

Sten Gustaf Stymne is a Swedish biochemist and professor emeritus in plant breeding at the Swedish University of Agricultural Sciences. During the years, Stymne has been one of the leaders in plant lipid biochemistry and was awarded the 2008 Terry Galliard Award as a recognition for his contributions to the field. Stymne has for many years been a frequent debater in various news media, criticizing Sweden and the European Union for its negative attitude towards plant biotechnology.

Diacylglycerol O-acyltransferase 2 (DGAT2) is a protein that in humans is encoded by the DGAT2 gene.
Pierre Benveniste, born on 22 December 1937 in Neuilly-sur-Seine, is a French researcher in plant biochemistry and professor at the University of Strasbourg.










