Related Research Articles

Histology, also known as microscopic anatomy or microanatomy, is the branch of biology that studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into organology, the study of organs, histology, the study of tissues, and cytology, the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms.

Haematoxylin or hematoxylin, also called natural black 1 or C.I. 75290, is a compound extracted from heartwood of the logwood tree with a chemical formula of C
16H
14O
6. This naturally derived dye has been used as a histologic stain, as an ink and as a dye in the textile and leather industry. As a dye, haematoxylin has been called palo de Campeche, logwood extract, bluewood and blackwood. In histology, haematoxylin staining is commonly followed by counterstaining with eosin. When paired, this staining procedure is known as H&E staining and is one of the most commonly used combinations in histology. In addition to its use in the H&E stain, haematoxylin is also a component of the Papanicolaou stain which is widely used in the study of cytology specimens.

Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ion, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2.

Alizarin is an organic compound with formula C
14H
8O
4 that has been used throughout history as a prominent red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus. In 1869, it became the first natural dye to be produced synthetically.

Tooth enamel is one of the four major tissues that make up the tooth in humans and many animals, including some species of fish. It makes up the normally visible part of the tooth, covering the crown. The other major tissues are dentin, cementum, and dental pulp. It is a very hard, white to off-white, highly mineralised substance that acts as a barrier to protect the tooth but can become susceptible to degradation, especially by acids from food and drink. In rare circumstances enamel fails to form, leaving the underlying dentin exposed on the surface.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.
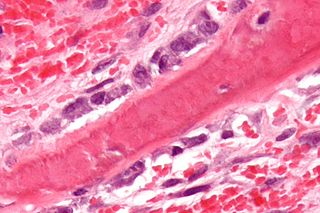
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

Histopathology refers to the microscopic examination of tissue in order to study the manifestations of disease. Specifically, in clinical medicine, histopathology refers to the examination of a biopsy or surgical specimen by a pathologist, after the specimen has been processed and histological sections have been placed onto glass slides. In contrast, cytopathology examines free cells or tissue micro-fragments.
Isotopic labeling is a technique used to track the passage of an isotope through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.
In pathology, silver staining is the use of silver to selectively alter the appearance of a target in microscopy of histological sections; in temperature gradient gel electrophoresis; and in polyacrylamide gels.

Hydroxyapatite is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), often written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. It is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride or chloride, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.

Tertiary hyperparathyroidism is a condition involving the overproduction of the hormone, parathyroid hormone, produced by the parathyroid glands. The parathyroid glands are involved in monitoring and regulating blood calcium levels and respond by either producing or ceasing to produce parathyroid hormone. Anatomically, these glands are located in the neck, para-lateral to the thyroid gland, which does not have any influence in the production of parathyroid hormone. Parathyroid hormone is released by the parathyroid glands in response to low blood calcium circulation. Persistent low levels of circulating calcium are thought to be the catalyst in the progressive development of adenoma, in the parathyroid glands resulting in primary hyperparathyroidism. While primary hyperparathyroidism is the most common form of this condition, secondary and tertiary are thought to result due to chronic kidney disease (CKD). Estimates of CKD prevalence in the global community range from 11 to 13% which translate to a large portion of the global population at risk of developing tertiary hyperparathyroidism. Tertiary hyperparathyroidism was first described in the late 1960s and had been misdiagnosed as primary prior to this. Unlike primary hyperparathyroidism, the tertiary form presents as a progressive stage of resolved secondary hyperparathyroidism with biochemical hallmarks that include elevated calcium ion levels in the blood, hypercalcemia, along with autonomous production of parathyroid hormone and adenoma in all four parathyroid glands. Upon diagnosis treatment of tertiary hyperparathyroidism usually leads to a surgical intervention.

Frank Burr Mallory was an American pathologist at the Boston City Hospital and Professor of Pathology at Harvard Medical School, after whom the Mallory body is named.
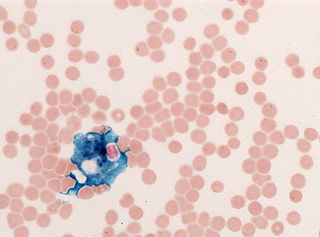
In histology, histopathology, and clinical pathology, Perls Prussian blue is a commonly used method to detect the presence of iron in tissue or cell samples. Perls Prussian Blue derives its name from the German pathologist Max Perls (1843–1881), who described the technique in 1867. The method does not involve the application of a dye, but rather causes the pigment Prussian blue to form directly within the tissue. The method stains mostly iron in the ferric state which includes ferritin and hemosiderin, rather than iron in the ferrous state.
Octacalcium phosphate (sometimes referred to as OCP) is a form of calcium phosphate with formula Ca8H2(PO4)6·5H2O. OCP may be a precursor to tooth enamel, dentine, and bones. OCP is a precursor of hydroxyapatite (HA), an inorganic biomineral that is important in bone growth. OCP has garnered lots of attention due to its inherent biocompatibility. While OCP exhibits good properties in terms of bone growth, very stringent synthesis requirements make it difficult for mass productions, but nevertheless has shown promise not only in-vitro, but also in in-vivo clinical case studies.
Trichrome stains are staining methods in which three anionic dyes are used, in conjunction with either phosphomolybdic acid (PMA), phosphotungstic acid (PTA), or a mixture of these heteropolyacids. Probably the first trichrome method was that of Frank B Mallory, an American pathologist, first published in 1900. Unfortunately, none of Mallory's publications provide any explanation of the rationales of either his trichrome or his phosphotungstic acid-haematoxylin (PTAH) method. Nobody knows why Mallory introduced heteropolyacids into microtechnique.

Silver phosphate or silver orthophosphate is a light sensitive, yellow, water-insoluble chemical compound composed of silver and phosphate ions of formula Ag3PO4.
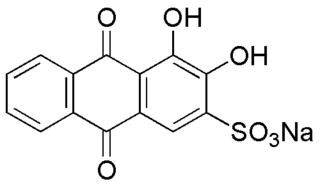
Alizarin Red S is a water-soluble sodium salt of Alizarin sulfonic acid with a chemical formula of C
14H
7NaO
7S. Alizarin Red S was discovered by Graebe and Libermann in 1871. In the field of histology alizarin Red S is used to stain calcium deposits in tissues, and in geology to stain and differentiate carbonate minerals.
References
- ↑ von Kossa J (1901) Ueber die im Organismus kunstlich erzeugbaren Verkalkungen. Beit Path Anat 29:163
- ↑ Clark G (1981) Staining Procedures. Williams and Wilkins, Baltimore, MD, pp 187
- ↑ Lillie R, Fuller H (1976) Histopathologic technique and practical histochemistry. McGraw-Hill, New York
- ↑ Mallory FB (1983) Pathological techniques: A practical manual for workers in pathological histology including directions for the performance of autopsies and for microphotography. In: Mallory NY (eds) WB Saunders, Philadelphia, PA, pp 143–144
- ↑ Drury, R. A. B.; Wallington, E. A. (1980). Carleton's Histological Technique (5th ed.). Oxford University Press. p. 520. ISBN 0-19-261310-3.
- ↑ Meloan, Susan N.; Puchtler, Holde (2013). "Chemical Mechanisms of Staining Methods: Von Kossa's Technique: What von Kossa Really Wrote and a Modified Reaction for Selective Demonstration of Inorganic Phosphates". Journal of Histotechnology. 8 (1): 11–13. doi:10.1179/his.1985.8.1.11. ISSN 0147-8885.