
A retrovirus is a type of RNA virus that inserts a copy of its genome into the DNA of a host cell that it invades, thus changing the genome of that cell. Once inside the host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus.

A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by retroviruses to replicate their genomes, by retrotransposon mobile genetic elements to proliferate within the host genome, by eukaryotic cells to extend the telomeres at the ends of their linear chromosomes, and by some non-retroviruses such as the hepatitis B virus, a member of the Hepadnaviridae, which are dsDNA-RT viruses.
Mouse mammary tumor virus (MMTV) is a milk-transmitted retrovirus like the HTL viruses, HI viruses, and BLV. It belongs to the genus Betaretrovirus. MMTV was formerly known as Bittner virus, and previously the "milk factor", referring to the extra-chromosomal vertical transmission of murine breast cancer by adoptive nursing, demonstrated in 1936, by John Joseph Bittner while working at the Jackson Laboratory in Bar Harbor, Maine. Bittner established the theory that a cancerous agent, or "milk factor", could be transmitted by cancerous mothers to young mice from a virus in their mother's milk. The majority of mammary tumors in mice are caused by mouse mammary tumor virus.
An oncovirus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.
Gammaretrovirus is a genus in the retroviridae family. Example species are the murine leukemia virus and the feline leukemia virus. They cause various sarcomas, leukemias and immune deficiencies in mammals, reptiles and birds.

Ribonuclease L or RNase L, known sometimes as ribonuclease 4 or 2'-5' oligoadenylate synthetase-dependent ribonuclease — is an interferon (IFN)-induced ribonuclease which, upon activation, destroys all RNA within the cell. RNase L is an enzyme that in humans is encoded by the RNASEL gene.
The murine leukemia viruses are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.
Jurkat cells are an immortalized line of human T lymphocyte cells that are used to study acute T cell leukemia, T cell signaling, and the expression of various chemokine receptors susceptible to viral entry, particularly HIV. Jurkat cells can produce interleukin 2, and are used in research involving the susceptibility of cancers to drugs and radiation.
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.

The human T-lymphotropic virus, human T-cell lymphotropic virus, or human T-cell leukemia-lymphoma virus (HTLV) family of viruses are a group of human retroviruses that are known to cause a type of cancer called adult T-cell leukemia/lymphoma and a demyelinating disease called HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP). The HTLVs belong to a larger group of primate T-lymphotropic viruses (PTLVs). Members of this family that infect humans are called HTLVs, and the ones that infect Old World monkeys are called Simian T-lymphotropic viruses (STLVs). To date, four types of HTLVs and four types of STLVs have been identified. HTLV types HTLV-1 and HTLV-2 viruses are the first retroviruses which were discovered. Both belong to the oncovirus subfamily of retroviruses and can transform human lymphocytes so that they are self-sustaining in vitro. The HTLVs are believed to originate from interspecies transmission of STLVs. The HTLV-1 genome is diploid, composed of two copies of a single-stranded RNA virus whose genome is copied into a double-stranded DNA form that integrates into the host cell genome, at which point the virus is referred to as a provirus. A closely related virus is bovine leukemia virus BLV. The original name for HIV, the virus that causes AIDS, was HTLV-3.
Whittemore Peterson Institute (WPI) currently located within the Center for Molecular Medicine at the University of Nevada, Reno, NV, was founded in 2005. WPI is a 501(c)3 Non-Profit medical research institute dedicated to scientific discovery surrounding complex neuroimmune diseases including myalgic encephalomyelitis (ME/CFS) and other similarly presenting illnesses.
Judy Anne Mikovits is a former American research scientist who is known for her discredited medical claims, such as that murine endogenous retroviruses are linked to chronic fatigue syndrome. She has been described as an anti-vaccination activist and a promoter of conspiracy theories, and has been accused of scientific misconduct. She has made several false claims about vaccines, COVID-19, and chronic fatigue syndrome (CFS).
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.
An endogenous viral element (EVE) is a DNA sequence derived from a virus, and present within the germline of a non-viral organism. EVEs may be entire viral genomes (proviruses), or fragments of viral genomes. They arise when a viral DNA sequence becomes integrated into the genome of a germ cell that goes on to produce a viable organism. The newly established EVE can be inherited from one generation to the next as an allele in the host species, and may even reach fixation.
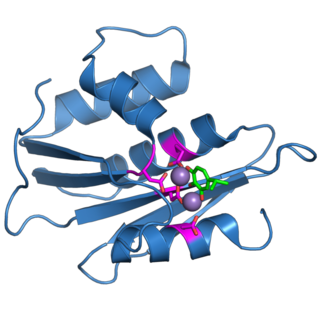
The retroviral ribonuclease H is a catalytic domain of the retroviral reverse transcriptase (RT) enzyme. The RT enzyme is used to generate complementary DNA (cDNA) from the retroviral RNA genome. This process is called reverse transcription. To complete this complex process, the retroviral RT enzymes need to adopt a multifunctional nature. They therefore possess 3 of the following biochemical activities: RNA-dependent DNA polymerase, ribonuclease H, and DNA-dependent DNA polymerase activities ). Like all RNase H enzymes, the retroviral RNase H domain cleaves DNA/RNA duplexes and will not degrade DNA or unhybridized RNA.
Lentiviral vectors in gene therapy is a method by which genes can be inserted, modified, or deleted in organisms using lentivirus.
Sandra L. Quackenbush is an American virologist working as an Associate Professor of Retrovirology at the Colorado State University College of Veterinary Medicine and Biomedical Sciences. Quackenbush also serves as the Associate Head of Graduate Education for the Microbiology, Immunology & Pathology Department within the college. Her research interests include viral pathogenesis, with emphasis in viral-induced oncogenesis.
VCaP cells are a cell line of human prostate cancer commonly used in the field of oncology. The tissue was harvested at autopsy from a metastatic lesion to a lumbar vertebrae of a 59 year old Caucasian male with hormone refractory prostate cancer in 1997, which was then xenografted into SCID mice and later harvested and plated on tissue culture dishes, where it can be propagated as an immortalized prostate cancer cell line.
Feline foamy virus or Feline syncytial virus is a retrovirus and belongs to the family Retroviridae and the subfamily Spumaretrovirinae. It shares the genus Felispumavirus with only Puma feline foamy virus. There has been controversy on whether FeFV is nonpathogenic as the virus is generally asymptomatic in affected cats and does not cause disease. However, some changes in kidney and lung tissue have been observed over time in cats affected with FeFV, which may or may not be directly affiliated. This virus is fairly common and infection rates gradually increase with a cat's age. Study results from antibody examinations and PCR analysis have shown that over 70% of felines over 9 years old were seropositive for Feline foamy virus. Viral infections are similar between male and female domesticated cats whereas in the wild, more feral females cats are affected with FeFV.
Gibbon-ape leukemia virus (GaLV) is an oncogenic, type C retrovirus that has been isolated from primate neoplasms, including the white-handed gibbon and woolly monkey.The virus was identified as the etiological agent of hematopoietic neoplasms, leukemias, and immune deficiencies within gibbons in 1971, during the epidemic of the late 1960s and early 1970's. Epidemiological research into the origins of GaLV has developed two hypothesises for the virus' emergence. These include; cross-species transmission of the retrovirus present within a species of East Asian rodent or bat, and the inoculation/ or blood transfusion of a MbRV-related virus into captured gibbons populations housed at medical research institutions. The virus was subsequently identified in captive gibbon populations in Thailand, the USA and Bermuda.





