
Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.
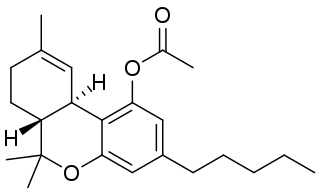
THC-O-acetate is the acetate ester of THC. The term THC-O-acetate and its variations are commonly used for two types of the substance, dependent on which cannabinoid it is synthesized from. The difference between Δ8-THC and Δ9-THC is bond placement on the cyclohexene ring.

Dimethylheptylpyran is a synthetic analog of THC, which was invented in 1949 during attempts to elucidate the structure of Δ9-THC, one of the active components of Cannabis. DMHP is a pale yellow, viscous oil which is insoluble in water but dissolves in alcohol or non-polar solvents.
Δ9-Tetrahydrocannabutol is a phytocannabinoid found in cannabis that is a homologue of tetrahydrocannabinol (THC), the main active component of Cannabis. Structurally, they are only different by the pentyl side chain being replaced by a butyl side chain. THCB was studied by Roger Adams as early as 1942
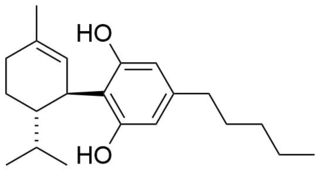
8,9-Dihydrocannabidiol is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity of 1.2 nM at CB1, approximately 33 times that of Δ9-THC (40 nM at CB1).

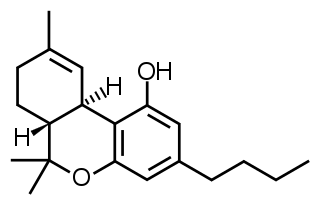
Δ-8-tetrahydrocannabinol is a psychoactive cannabinoid found in the Cannabis plant. It is an isomer of delta-9-tetrahydrocannabinol, the compound commonly known as THC, with which it co-occurs in hemp; natural quantities of ∆8-THC found in hemp are low.

Δ-10-Tetrahydrocannabinol is a positional isomer of tetrahydrocannabinol, discovered in the 1980s. Two enantiomers have been reported in the literature, with the 9-methyl group in either the (R) or (S) conformation; of these, the (R) enantiomer appears to be the more active isomer as well as the double bond in the 10th position instead of the 9th maintaining about 30 to 40 percent the potency of delta-9-THC. Δ10-THC has rarely been reported as a trace component of natural cannabis, though it is thought to be a degradation product similar to cannabinol rather than being produced by the plant directly. However, it is found more commonly as an impurity in synthetic delta-8-THC produced from cannabidiol and can also be synthesized directly from delta-9-THC.

Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by acid cyclization and hydrogenation of tetrahydrocannabinol. The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams.

11-Hydroxyhexahydrocannabinol is an active metabolite of tetrahydrocannabinol (THC) and a metabolite of the trace cannabinoid hexahydrocannabinol (HHC).

Tetrahydrocannabihexol is a phytocannabinoid, the hexyl homologue of tetrahydrocannabinol (THC) which was first isolated from Cannabis plant material in 2020 along with the corresponding hexyl homologue of cannabidiol, though it had been known for several decades prior to this as an isomer of the synthetic cannabinoid parahexyl. Another isomer Δ8-THCH is also known as a synthetic cannabinoid under the code number JWH-124, though it is unclear whether this occurs naturally in Cannabis, but likely is due to Δ8-THC itself being a degraded form of Δ9-THC. THC-Hexyl can be synthesized from 4-hexylresorcinol and was studied by Roger Adams as early as 1942.
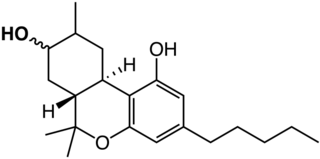
8-Hydroxyhexahydrocannabinols are active primary metabolites of hexahydrocannabinol (HHC) in animals and trace phytocannabinoids. The 8-OH-HHCs are produced in notable concentrations following HHC administration in several animal species, including humans. They have drawn research interest for their role in HHC toxicology and stereoisomeric probes of the cannabinoid drug/receptor interaction.

HHC-acetate is a semi-synthetic cannabinoid derivative which has been marketed since around 2022. It is believed to be made in a three step process from cannabidiol extracted from hemp. The legal status of hexahydrocannabinol and derivatives such as HHC-O varies between countries leading to widespread sale in some jurisdictions in Europe and the US, but in France HHC and HHC-O were banned in 2023, and HHC is already banned in several other countries. On 1st of March 2024 HHC was banned on Czech Republic.

Hexahydrocannabihexol (HHCH) is a semi-synthetic cannabinoid derivative. It was first synthesised by Roger Adams in 1942 and found to be more potent than either the pentyl or heptyl homologues, or the unsaturated tetrahydrocannabinol analogue.
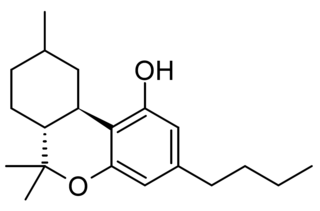
Hexahydrocannabutol is a semi-synthetic cannabinoid derivative, the hydrogenated derivative of tetrahydrocannabutol (THCB). It was first synthesised by Roger Adams in 1942 and produces only weak cannabinoid-like effects in animals. More recently it has been sold as an ingredient in grey-market cannabinoid products.
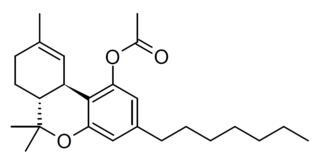
THCP-O-acetate (THCP-O) is a semi-synthetic derivative of tetrahydrocannabiphorol (THCP) derived by acetylation of the OH group. It has been found as a component of grey-market cannabis products such as e-cigarette liquids and edible gummy lollies, and is allegedly a potent and long-lasting psychoactive cannabinoid.
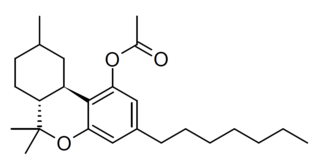
HHCP-O-acetate is a semi-synthetic derivative of tetrahydrocannabiphorol (THCP) derived in several steps by hydrogenation to hexahydrocannabiphorol (HHCP) followed by acetylation of the OH group. It has been found as a component of grey-market cannabis products such as e-cigarette liquids and edible gumdrops, and is allegedly a potent and long-lasting psychoactive cannabinoid.
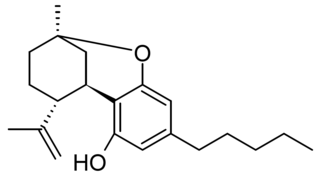
Isotetrahydrocannabinol (iso-THC) is a phytocannabinoid similar in structure to cannabicitran which has been identified as a trace component of Cannabis, but is more commonly found as an impurity in synthetic THC which has been made from cannabidiol, along with other isomers with the double bond in a different position and the saturated dihydro derivative. iso-THC can be described as the upper cyclization product of CBD, while THC is the lower cyclization product of CBD. Its pharmacology has not been studied, though it is commonly found as a trace impurity in commercially marketed Δ8-THC products.

Abeo-HHC acetate is a semi-synthetic derivative of tetrahydrocannabinol, first described in the 1980s. It is synthesised from delta-11-tetrahydrocannabinol, which can be made to undergo a ring expansion reaction via a hydrazone intermediate. It is structurally similar to HHC-acetate except that the methylated cyclohexyl ring has been replaced by a cycloheptane.




















