
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

In biochemistry, a cholinesterase or choline esterase is a family of esterases that lyses choline-based esters, several of which serve as neurotransmitters. Thus, it is either of two enzymes that catalyze the hydrolysis of these cholinergic neurotransmitters, such as breaking acetylcholine into choline and acetic acid. These reactions are necessary to allow a cholinergic neuron to return to its resting state after activation. For example, in muscle contraction, acetylcholine at a neuromuscular junction triggers a contraction; but for the muscle to relax afterward, rather than remaining locked in a tense state, the acetylcholine must be broken down by a choline esterase. The main type for that purpose is acetylcholinesterase ; it is found mainly in chemical synapses and red blood cell membranes. The other type is butyrylcholinesterase ; it is found mainly in the blood plasma.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. Common uses of parasympathomimetics include glaucoma, sjögren syndrome and underactive bladder.

Galantamine is used for the treatment of cognitive decline in mild to moderate Alzheimer's disease and various other memory impairments. It is an alkaloid that has been isolated from the bulbs and flowers of Galanthus nivalis, Galanthus caucasicus, Galanthus woronowii, and some other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata. It can also be produced synthetically.

Neuromuscular-blocking drugs block neuromuscular transmission at the neuromuscular junction, causing paralysis of the affected skeletal muscles. This is accomplished via their action on the post-synaptic acetylcholine (Nm) receptors.
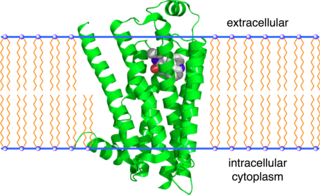
The beta-2 adrenergic receptor, also known as ADRB2, is a cell membrane-spanning beta-adrenergic receptor that binds epinephrine (adrenaline), a hormone and neurotransmitter whose signaling, via adenylate cyclase stimulation through trimeric Gs proteins, increased cAMP, and downstream L-type calcium channel interaction, mediates physiologic responses such as smooth muscle relaxation and bronchodilation.

Zomepirac is an orally effective nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic actions. It was developed by McNeil Pharmaceutical, approved by the FDA in 1980, and sold as the sodium salt zomepirac sodium, under the brand name Zomax. Due to its clinical effectiveness, it was preferred by doctors in many situations and obtained a large share of the analgesics market; however, it was subsequently withdrawn in March 1983 due to its tendency to cause serious anaphylaxis in a small, but unpredictable, subset of the patient population.

Acetylcholinesterase, also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters. AChE is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides.

SH3 and multiple ankyrin repeat domains protein 1 is a protein that in humans is encoded by the SHANK1 gene.

Diclofensine was developed by Hoffmann-La Roche in the 1970s in the search for a new antidepressant. It was found that the (S)-isomer was responsible for activity. Is a stimulant drug which acts as a triple monoamine reuptake inhibitor, primarily inhibiting the reuptake of dopamine and norepinephrine, with affinities (Ki) of 16.8 nM, 15.7 nM, and 51 nM for DAT, NET, and SERT, respectively. It was found to be an effective antidepressant in human trials, with relatively few side effects, but was ultimately dropped from clinical development, possibly due to concerns about its abuse potential.
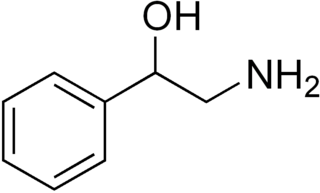
Phenylethanolamine, or β-hydroxyphenethylamine, is a trace amine with a structure similar to those of other trace phenethylamines as well as the catecholamine neurotransmitters dopamine, norepinephrine, and epinephrine. As an organic compound, phenylethanolamine is a β-hydroxylated phenethylamine that is also structurally related to a number of synthetic drugs in the substituted phenethylamine class. In common with these compounds, phenylethanolamine has strong cardiovascular activity and, under the name Apophedrin, has been used as a drug to produce topical vasoconstriction.

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.

G-130 is a drug with stimulant and anorectic effects, related to phenmetrazine.

Leptophos belongs to the organophosphates and at room temperature it is a stable white solid. It is also known as Phosvel, Abar and Vcs 506. Leptophos was primarily used as a pesticide and fungicide. for rice, cotton, fruit and vegetables until its use was discontinued in 1975 in USA, but still sold in South-Eastern Asia in 1981.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase (AChE), the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.
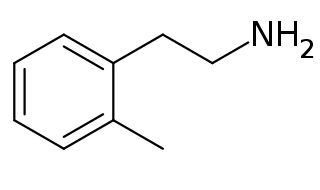
2-Methylphenethylamine (2MPEA) is an organic compound with the chemical formula of C9H13N. 2MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine.

3-Methylphenethylamine (3MPEA) is an organic compound with the chemical formula of C9H13N. 3MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine.

4-Methylphenethylamine (4MPEA), also known as para-methylphenethylamine, is an organic compound with the chemical formula of C9H13N. 4MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine. 4MPEA also appears to inhibit the human cytochrome P450 enzymes CYP1A2 and CYP2A6, based upon the published literature.
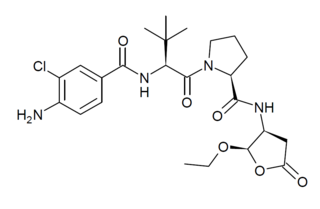
Belnacasan (VX-765) is a drug developed by Vertex Pharmaceuticals which acts as a potent and selective inhibitor of the enzyme caspase 1. This enzyme is involved in inflammation and cell death, and consequently blocking its action may be useful for various medical applications, including treatment of epilepsy, arthritis, aiding recovery from heart attack and slowing the progression of Alzheimer's disease. Belnacasan is an orally active prodrug, being converted in the body to the active drug VRT-043198 (O-desethyl-belnacasan). However while belnacasan has proved well tolerated in human clinical trials, it has not shown sufficient efficacy to be approved for use for any of the applications suggested to date, though research continues into possible future uses of this or similar drugs.
BW284C51 is a selective acetylcholinesterase inhibitor. It is also a nicotinic antagonist.

















