Related Research Articles

Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.
Endocrine disruptors, sometimes also referred to as hormonally active agents, endocrine disrupting chemicals, or endocrine disrupting compounds are chemicals that can interfere with endocrine systems. These disruptions can cause cancerous tumors, birth defects, and other developmental disorders. Found in many household and industrial products, endocrine disruptors "interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for development, behavior, fertility, and maintenance of homeostasis ."
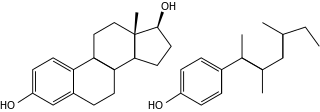
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estrogenic effects on a living organism even though they differ chemically from the estrogenic substances produced internally by the endocrine system of any organism. Natural xenoestrogens include phytoestrogens which are plant-derived xenoestrogens. Because the primary route of exposure to these compounds is by consumption of phytoestrogenic plants, they are sometimes called "dietary estrogens". Mycoestrogens, estrogenic substances from fungi, are another type of xenoestrogen that are also considered mycotoxins.
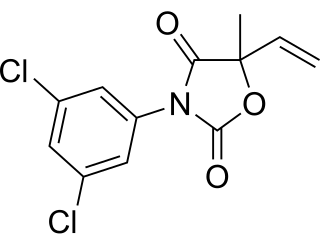
Vinclozolin is a common dicarboximide fungicide used to control diseases, such as blights, rots and molds in vineyards, and on fruits and vegetables such as raspberries, lettuce, kiwi, snap beans, and onions. It is also used on turf on golf courses. Two common fungi that vinclozolin is used to protect crops against are Botrytis cinerea and Sclerotinia sclerotiorum. First registered in 1981, vinclozolin is widely used but its overall application has declined. As a pesticide, vinclozolin is regulated by the United States Environmental Protection Agency. In addition to these restrictions within the United States, as of 2006 the use of this pesticide was banned in several countries, including Denmark, Finland, Norway, and Sweden. It has gone through a series of tests and regulations in order to evaluate the risks and hazards to the environment and animals. Among the research, a main finding is that vinclozolin has been shown to be an endocrine disruptor with antiandrogenic effects.
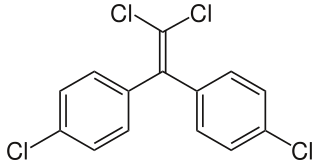
Dichlorodiphenyldichloroethylene (DDE) is a chemical compound formed by the loss of hydrogen chloride (dehydrohalogenation) from DDT, of which it is one of the more common breakdown products. Due to DDT's massive prevalence in society and agriculture during the mid 20th century, DDT and DDE are still widely seen in animal tissue samples. DDE is particularly dangerous because it is fat-soluble like other organochlorines; thus, it is rarely excreted from the body, and concentrations tend to increase throughout life. The major exception is the excretion of DDE in breast milk, which transfers a substantial portion of the mother's DDE burden to the young animal or child. Along with accumulation over an organism's lifetime, this stability leads to bioaccumulation in the environment, which amplifies DDE's negative effects.

Dicarboximidefungicides are a family of agricultural fungicides that include vinclozolin, iprodione, and procymidone. Dicarboximides are believed to inhibit triglyceride biosynthesis in sclerotia-forming fungi, including Botrytis cinerea. These fungicides turn into 3,5-dichloroaniline in soil rapidly. Repeated use of dicarboximides over several years reduce their effectiveness. Resistance has developed against all dicarboximides in many plant species, including vines, strawberries and protected crops, and are recommended to be used in conjunction with other fungicides.

Procymidone is a pesticide. It is often used for killing unwanted ferns and nettles, and as a dicarboximide fungicide for killing fungi, for example as seed dressing, pre-harvest spray or post-harvest dip of lupins, grapes, stone fruit, strawberries. It is a known endocrine disruptor which interferes with the sexual differention of male rats. It is considered to be a poison.

Diethyl phthalate (DEP) is a phthalate ester. It occurs as a colourless liquid without significant odour but has a bitter, disagreeable taste. It is more dense than water and insoluble in water; hence, it sinks in water.
This article is about the discovery and development of antiandrogens, or androgen receptor (AR) antagonists.

A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity.

A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone.

BOMT, also known by its developmental code name Ro 7-2340 and as 6α-bromo-4-oxa-17α-methyl-5α-dihydrotestosterone, is a synthetic steroidal antiandrogen which was first developed in 1970 and was never marketed for medical use. It is the 6α-brominated, 4-oxygenated, and 17α-methylated derivative of the androgen dihydrotestosterone (DHT). Along with benorterone, cyproterone, and flutamide, BOMT was among the earliest antiandrogens to be developed and extensively studied, although it is less well-documented in comparison to the others. BOMT has been investigated clinically in the treatment of benign prostatic hyperplasia, though development for this use did not continue. There was also interest in BOMT for the potential applications of acne, pattern hair loss, and possibly prostate cancer, but it was not developed for these indications either.
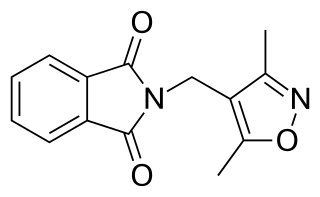
DIMP, or N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide, is a nonsteroidal antiandrogen (NSAA) structurally related to thalidomide that was first described in 1973 and was never marketed. Along with flutamide, it was one of the earliest NSAAs to be discovered, and for this reason, has been described as a "classical" NSAA. The drug is a selective, competitive, silent antagonist of the AR, although it is described as an "only relatively weak competitor". Its relative binding affinity for the androgen receptor is about 2.6% of that of metribolone. DIMP possesses no androgenic, estrogenic, progestogenic, or antigonadotropic activity, but it does reverse the antigonadotropic effects of testosterone, indicating that, like other pure AR antagonists, it is progonadotropic.

Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.

AA560 is an orally active nonsteroidal antiandrogen (NSAA) that was developed in Japan and was first described in the literature in 1977 but was never marketed. It is an anilide derivative and analogue of the NSAA flutamide, and shows greater in vivo antiandrogenic potency than does flutamide. Similarly to flutamide, AA560 is a selective antagonist of the androgen receptor (AR) and consequently shows progonadotropic effects by increasing levels of gonadotropins and testosterone via disinhibition of the hypothalamic-pituitary-gonadal axis.

Prochloraz, brand name Sportak, is an imidazole fungicide that was introduced in 1978 and is widely used in Europe, Australia, Asia, and South America within gardening and agriculture to control the growth of fungi. It is not registered for use in the United States. Similarly to other azole fungicides, prochloraz is an inhibitor of the enzyme lanosterol 14α-demethylase (CYP51A1), which is necessary for the production of ergosterol – an essential component of the fungal cell membrane – from lanosterol. The agent is a broad-spectrum, protective and curative fungicide, effective against Alternaria spp., Botrytis spp., Erysiphe spp., Helminthosporium spp., Fusarium spp., Pseudocerosporella spp., Pyrenophora spp., Rhynchosporium spp., and Septoria spp.

The pharmacology of bicalutamide, a nonsteroidal antiandrogen (NSAA), has been well-characterized. In terms of pharmacodynamics, bicalutamide acts as a selective antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has no capacity to activate the AR. It does not decrease androgen levels and has no other important hormonal activity. The medication has progonadotropic effects due to its AR antagonist activity and can increase androgen, estrogen, and neurosteroid production and levels. This results in a variety of differences of bicalutamide monotherapy compared to surgical and medical castration, such as indirect estrogenic effects and associated benefits like preservation of sexual function and drawbacks like gynecomastia. Bicalutamide can paradoxically stimulate late-stage prostate cancer due to accumulated mutations in the cancer. When used as a monotherapy, bicalutamide can induce breast development in males due to its estrogenic effects. Unlike other kinds of antiandrogens, it may have less adverse effect on the testes and fertility.
Dimethylcurcumin is a nonsteroidal antiandrogen and a synthetic curcuminoid which is under development by AndroScience Corporation as a topical medication for the treatment of acne vulgaris. It has also been under investigation for the treatment of male pattern hair loss, spinal muscular atrophy, and wounds, but no development has been reported for these indications. There has been interest in the drug for the potential treatment of prostate cancer as well. As of 2017, it is in phase II clinical trials for acne vulgaris.

BMS-641988 is a nonsteroidal antiandrogen which was developed by Bristol-Myers Squibb for the treatment of prostate cancer but was never marketed. It acts as a potent competitive antagonist of the androgen receptor (AR) (Ki = 10 nM; IC50 = 56 nM). The drug was found to have 20-fold higher affinity for the AR than bicalutamide in MDA-MB-453 cells, and showed 3- to 7-fold the antiandrogenic activity of bicalutamide in vitro. It may have some weak partial agonist activity at the androgen receptor. BMS-641988 is transformed by CYP3A4 into BMS-570511, and this metabolite is then reduced to BMS-501949 by cytosolic reductases. All three compounds show similar antiandrogenic activity. In addition to its antiandrogenic activity, BMS-641988 shows activity as a negative allosteric modulator of the GABAA receptor, and can produce seizures in animals at sufficiently high doses. It also shows some drug-induced QT prolongation. BMS-641988 reached phase I clinical trials prior to the discontinuation of its development. The clinical development of BMS-641988 was terminated due to the occurrence of a seizure in a patient during a phase I study.

The pharmacology of cyproterone acetate (CPA) concerns the pharmacology of the steroidal antiandrogen and progestin medication cyproterone acetate.
References
- 1 2 3 4 5 6 7 8 9 10 Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L (2001). "Effects of environmental antiandrogens on reproductive development in experimental animals". Human Reproduction Update. 2 (3): 248–64. doi: 10.1093/humupd/7.3.248 . PMID 11392371.
- 1 2 3 4 Rider CV, Furr JR, Wilson VS, Gray LE Jr (Apr 2010). "Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity". International Journal of Andrology. 33 (2): 443–62. doi:10.1111/j.1365-2605.2009.01049.x. PMC 2874988 . PMID 20487044.
- ↑ Curtis LR (Mar 2001). "Organophosphate antagonism of the androgen receptor". Toxicological Sciences . 60 (1): 1–2. doi: 10.1093/toxsci/60.1.1 . PMID 11222865.
- ↑ Barros, Alfredo Carlos Simões Dornellas de; Sampaio, Marcelo de Castro Moura (2012). "Gynecomastia: physiopathology, evaluation and treatment". Sao Paulo Medical Journal. 130 (3): 187–197. doi: 10.1590/S1516-31802012000300009 . ISSN 1516-3180. PMID 22790552.
Reinforcing the evidence suggesting that there is a relationship between chemicals and GM, it is worthwhile mentioning the epidemic onset observed among Haitian refugees in 1981 about four months after arrival in United States detention centers.22 After analyzing all identifiable environmental exposures, it was then found that phenothrin, a multi-insecticide contained in sprays that they had used was the causative agent.23 It is now widely known that phenothrin has antiandrogenic activity.
- 1 2 Darbre PD, Harvey PW (Jul 2008). "Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks". Journal of Applied Toxicology. 28 (5): 561–78. doi: 10.1002/jat.1358 . PMID 18484575.
- ↑ Yang, Sarah (12 May 2003). "Chemical in Broccoli Blocks Growth of Prostate Cancer Cells, New Study Shows" . Retrieved 8 April 2015.
- ↑ Le, H. T.; Schaldach, C. M.; Firestone, G. L.; Bjeldanes, L. F. (6 June 2003). "Plant-derived 3,3'-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells". J Biol Chem. 278 (23): 21136–45. doi: 10.1074/jbc.M300588200 . PMID 12665522.
- ↑ Papaioannou M, Schleich S, Roell D, Schubert U, Tanner T, Claessens F, Matusch R, Baniahmad A (December 2010). "NBBS isolated from Pygeum africanum bark exhibits androgen antagonistic activity, inhibits AR nuclear translocation and prostate cancer cell growth". Invest New Drugs. 28 (6): 729–43. doi:10.1007/s10637-009-9304-y. PMID 19771394. S2CID 5455114.
- ↑ Schleich S, Papaioannou M, Baniahmad A, Matusch R (July 2006). "Extracts from Pygeum africanum and other ethnobotanical species with antiandrogenic activity". Planta Med. 72 (9): 807–13. doi:10.1055/s-2006-946638. PMID 16783690.
- ↑ Zamansoltani F, Nassiri-Asl M, Sarookhani MR, Jahani-Hashemi H, Zangivand AA (August 2009). "Antiandrogenic activities of Glycyrrhiza glabra in male rats". Int. J. Androl. 32 (4): 417–22. doi: 10.1111/j.1365-2605.2008.00944.x . PMID 19515171.