Related Research Articles
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula CH3Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It is a recognized ozone-depleting chemical. It was used extensively as a pesticide until being phased out by most countries in the early 2000s. From the chemistry perspective, it is one of the halomethanes.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
IARC group 3 substances, chemical mixtures and exposure circumstances are those that can not be classified in regard to their carcinogenicity to humans by the International Agency for Research on Cancer (IARC). This category is used most commonly for agents, mixtures and exposure circumstances for which the level of evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans, but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid described by the formula CH3CH=CHCO2H. The name crotonic acid was given because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. With a cis-alkene, Isocrotonic acid is an isomer of crotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to that of butyric acid.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs.
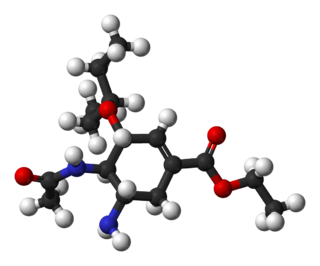
Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug oseltamivir marketed by Hoffmann-La Roche under the trade name Tamiflu. Its commercial production starts from the biomolecule shikimic acid harvested from Chinese star anise and from recombinant E. coli. Control of stereochemistry is important: the molecule has three stereocenters and the sought-after isomer is only 1 of 8 stereoisomers.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
This is an index of articles relating to pesticides.
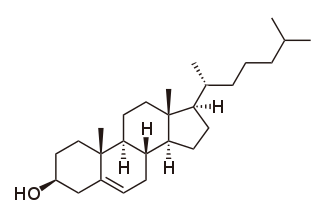
Cholesterol total synthesis in chemistry describes the total synthesis of the complex biomolecule cholesterol and is considered a great scientific achievement. The research group of Robert Robinson with John Cornforth published their synthesis in 1951 and that of Robert Burns Woodward with Franz Sondheimer in 1952. Both groups competed for the first publication since 1950 with Robinson having started in 1932 and Woodward in 1949. According to historian Greg Mulheirn the Robinson effort was hampered by his micromanagement style of leadership and the Woodward effort was greatly facilitated by his good relationships with chemical industry. Around 1949 steroids like cortisone were produced from natural resources but expensive. Chemical companies Merck & Co. and Monsanto saw commercial opportunities for steroid synthesis and not only funded Woodward but also provided him with large quantities of certain chemical intermediates from pilot plants. Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.

Tefluthrin is the ISO common name for an organic compound that is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as tefluthrin are often preferred as active ingredients in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. It is effective against soil pests because it can move as a vapour without irreversibly binding to soil particles: in this respect it differs from most other pyrethroids.
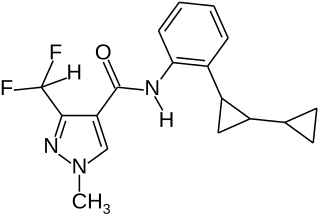
Sedaxane is a broad spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2011 using their brand name Vibrance. The compound is an amide which combines a pyrazole acid with an aryl amine to give an inhibitor of succinate dehydrogenase.
11-Aminoundecanoic acid is an organic compound with the formula H2N(CH2)10CO2H. This white solid is classified as an amine and a fatty acid. 11-Aminoundecanoic acid is a precursor to Nylon-11.
References
- ↑ "Compendium of Pesticide Common Names: Fungicides". BCPC. Retrieved 2023-08-13.
- ↑ Lewis, Kathleen A.; Tzilivakis, John; Warner, Douglas J.; Green, Andrew (2016). "An international database for pesticide risk assessments and management". Human and Ecological Risk Assessment. 22 (4): 1050–1064. doi:10.1080/10807039.2015.1133242. hdl: 2299/17565 . S2CID 87599872.
- ↑ "A to Z List of Fungicides". University of Hertfordshire. 2023-08-08. Retrieved 2023-08-13.
- ↑ "FRAC Recommendations for Resistance Management". Fungicides Resistance Action Committee. Retrieved 2023-08-13.
- ↑ "FRAC Classification of Fungicides" (PDF). CropLife International. 2022-04-21.
- ↑ Bentley, Ronald (2008). "A Fresh Look at Natural Tropolonoids". Natural Product Reports. 25 (1): 118–138. doi:10.1039/B711474E. PMID 18250899.