Triple-negative breast cancer (TNBC) is any breast cancer that either lacks or shows low levels of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) overexpression and/or gene amplification. Triple-negative is sometimes used as a surrogate term for basal-like.
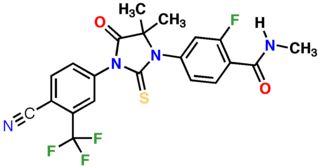
Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.

Olaparib, sold under the brand name Lynparza, is a medication for the maintenance treatment of BRCA-mutated advanced ovarian cancer in adults. It is a PARP inhibitor, inhibiting poly ADP ribose polymerase (PARP), an enzyme involved in DNA repair. It acts against cancers in people with hereditary BRCA1 or BRCA2 mutations, which include some ovarian, breast, and prostate cancers.

PARP inhibitors are a group of pharmacological inhibitors of the enzyme poly ADP ribose polymerase (PARP).

Iniparib was a drug candidate for cancer treatment. It was originally believed to act as an irreversible inhibitor of PARP1 and possibly other enzymes through covalent modification, but its effects against PARP were later disproven. It underwent clinical trials for treatment of some types of breast cancer, but was discontinued after disappointing phase III clinical trials.

Veliparib (ABT-888) is a potential anti-cancer drug acting as a PARP inhibitor. It kills cancer cells by blocking a protein called PARP, thereby preventing the repair of DNA or genetic damage in cancer cells and possibly making them more susceptible to anticancer treatments. Veliparib may make whole brain radiation treatment work more effectively against brain metastases from NSCLC. It has been shown to potentiate the effects of many chemotherapeutics, and as such has been part of many combination clinical trials.

Rucaparib, sold under the brand name Rubraca, is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It is taken by mouth.
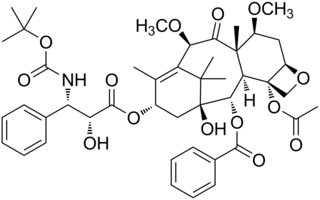
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Medivation was an American biopharmaceutical company focused on development of novel therapies to treat serious diseases for which there are limited treatment options. Medivation was headquartered in San Francisco, California, beginning operations in December 2004 with the acquisition of Medivation Neurology, Inc. Its final CEO was David Hung.

Seviteronel is an experimental cancer medication which is under development by Viamet Pharmaceuticals and Innocrin Pharmaceuticals for the treatment of prostate cancer and breast cancer. It is a nonsteroidal CYP17A1 inhibitor and works by inhibiting the production of androgens and estrogens in the body. As of July 2017, seviteronel is in phase II clinical trials for both prostate cancer and breast cancer. In January 2016, it was designated fast-track status by the United States Food and Drug Administration for prostate cancer. In April 2017, seviteronel received fast-track designation for breast cancer as well.

Sacituzumab govitecan, sold under the brand name Trodelvy, is a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate used for the treatment of metastatic triple-negative breast cancer and metastatic urothelial cancer.

Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.
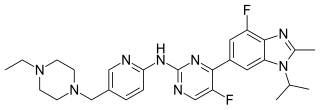
Abemaciclib, sold under the brand name Verzenio among others, is a medication for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.
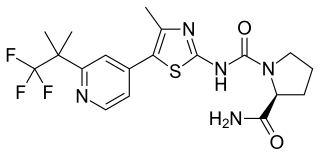
Alpelisib, sold under the brand name Piqray among others, is a medication used to treat certain types of breast cancer. It is used together with fulvestrant. It is taken by mouth. It is marketed by Novartis.

Niraparib, sold under the brand name Zejula, is an anti-cancer medication used for the treatment of epithelial ovarian, fallopian tube, or primary peritoneal cancer. It is taken by mouth. It is a PARP inhibitor.

Elacestrant, sold under the brand name Orserdu, is an anticancer medication which is used in the treatment of breast cancer. It is taken by mouth.
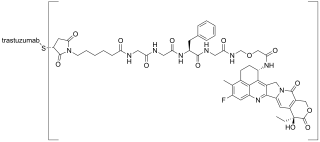
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.
Pertuzumab/trastuzumab/hyaluronidase, sold under the brand name Phesgo, is a fixed-dose combination medication to treat adults with HER2-positive breast cancer that has spread to other parts of the body, and for treatment of adults with early HER2-positive breast cancer. It contains pertuzumab, trastuzumab, and hyaluronidase–zzxf. It is injected under the skin via subcutaneous injection in the thigh. In the European Union, Phesgo contains the active ingredients pertuzumab and trastuzumab along with the enzyme vorhyaluronidase alfa.
Niraparib/abiraterone acetate, sold under the brand name Akeega, is a fixed-dose combination anti-cancer medication used for the treatment of prostate cancer. It contains niraparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, and abiraterone acetate, a CYP17 inhibitor.

Capivasertib, sold under the brand name Truqap, is an anti-cancer medication used for the treatment of breast cancer. It is taken by mouth.
















