Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania

Evofosfamide is an investigational hypoxia-activated prodrug that is in clinical development for cancer treatment. The prodrug is activated only at very low levels of oxygen (hypoxia). Such levels are common in human solid tumors, a phenomenon known as tumor hypoxia.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.
Farletuzumab (MORAb-003) is a humanized monoclonal antibody of IgG1/κ which is being investigated for the treatment of ovarian cancer.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.
Figitumumab is a monoclonal antibody targeting the insulin-like growth factor-1 receptor that was investigated for the treatment of various types of cancer, for example adrenocortical carcinoma and non-small cell lung cancer (NSCLC).
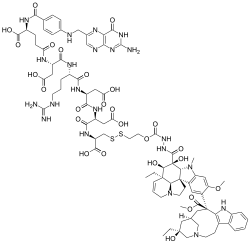
Folate targeting is a method utilized in biotechnology for drug delivery purposes. This Trojan Horse process, which was created by Drs. Christopher P. Leamon and Philip S. Low, involves the attachment of the vitamin, folate, to a molecule/drug to form a "folate conjugate". Based on the natural high affinity of folate for the folate receptor protein (FR), which is commonly expressed on the surface of many human cancers, folate-drug conjugates also bind tightly to the FR and trigger cellular uptake via endocytosis. Molecules as diverse as small radiodiagnostic imaging agents to large DNA plasmid formulations have successfully been delivered inside FR-positive cells and tissues.
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seattle Genetics in the US.
Tecemotide is a synthetic lipopeptide that is used as antigen in an investigational therapeutic cancer vaccine. The investigational therapeutic cancer vaccine is designed to induce a cellular immune response to cancer cells that express MUC1, a glycoprotein antigen that is widely over-expressed on common cancers such as lung cancer, breast cancer, prostate cancer, and colorectal cancer. The cellular immune response may lead to a rejection of tumor tissue expressing the MUC1 antigen.

Zoptarelin doxorubicin consists of doxorubicin linked to a small peptide agonist to the luteinizing hormone-releasing hormone (LHRH) receptor. It has been developed as a potential treatment for a number of human cancers. The LHRH receptor is aberrantly present on the cell surface of approximately 80% of endometrial and ovarian cancers, 86% of prostate cancers and about 50% of breast cancers. Whereas in normal tissues, expression of this receptor is mainly confined to the pituitary gland, reproductive organs and hematopoietic stem cells. To a lesser extent the LHRH receptor is also found on the surface of bladder, colorectal, and pancreatic cancers, sarcomas, lymphomas, melanomas, and renal cell carcinomas.
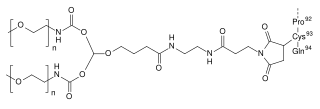
Pegdinetanib is an investigational anti-cancer drug that acts as a selective antagonist of vascular endothelial growth factor receptor 2 (VEGFR-2), hindering vascularization of tumors. It is a genetically engineered peptide derivative based on the monobody technology, and is being developed by Adnexus.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is given by slow injection into a vein.
Endocyte is a biopharmaceutical company established in 1996 and headquartered in West Lafayette, Indiana, a resident of the Purdue Research Park. In 2011 the company completed successfully an initial public offering (IPO). As of 2013, the company had 93 employees. The original president and CEO, Ron Ellis, was succeeded by Mike Sherman, who held a CFO position at the company before this change in June 2016. In 2018 the company was acquired by Novartis.

Technetium (99mTc) etarfolatide is an investigational non-invasive, folate receptor-targeting companion imaging agent that is being developed by Endocyte. Etarfolatide consists of a small molecule targeting the folate receptor and an imaging agent, which is based on technetium-99m. This companion imaging agent identifies cells expressing the folate receptor, including cancer and inflammatory cells.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
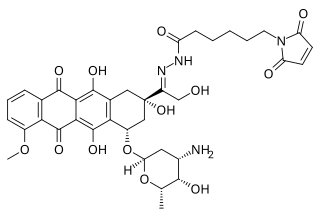
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid-sensitive linker.
Avelumab, sold under the brand name Bavencio, is a fully human monoclonal antibody medication for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma.
Cofetuzumab pelidotin is an experimental antibody-drug conjugate in development for the treatment of cancer. It was created by Stemcentrx and is being developed by Pfizer. The drug is an anti-PTK7 monoclonal antibody linked to auristatin-0101, an auristatin microtubule inhibitor.