Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.

The organic compound 1,1,1-trichloroethane, also known as methyl chloroform and chlorothene, is a chloroalkane with the chemical formula CH3CCl3. It is an isomer of 1,1,2-trichloroethane. This colorless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2021, it was the 291st most commonly prescribed medication in the United States, with more than 600,000 prescriptions. It is also used in metallurgy and in medical imaging.
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colorless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature. CFC-11 is a Class 1 ozone-depleting substance which damages Earth's protective stratospheric ozone layer.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.

Nitrogen trifluoride is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century.

Chromium(III) fluoride is an inorganic compound with the chemical formula CrF3. It forms several hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the hydrates [Cr(H2O)6]F3 (violet) and [Cr(H2O)6]F3·3H2O (green) are soluble in water. The anhydrous form sublimes at 1100–1200 °C.
1,1-Dichloroethane is a chlorinated hydrocarbon. It is a colorless oily liquid with a chloroform-like odor. It is not easily soluble in water, but miscible with most organic solvents.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, much higher than other hydrogen halides.
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents. 1,1-DCE was the precursor to the original clingwrap, Saran, for food, but this application has been phased out.
1,1,2-Trichloroethane, or 1,1,2-TCA, is an organochloride solvent with the molecular formula C2H3Cl3 and the structural formula CH2Cl—CHCl2. It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents. It is an isomer of 1,1,1-trichloroethane, and a byproduct of its manufacture.

Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds.

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Aluminium fluoride is an inorganic compound with the formula AlF3. It forms hydrates AlF3·xH2O. Anhydrous AlF3 and its hydrates are all colorless solids. Anhydrous AlF3 is used in the production of aluminium. Several occur as minerals.
Carbonyl fluoride is a chemical compound with the formula COF2. It is a carbon oxohalide. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with C2v symmetry, bond lengths of 1.174 Å (C=O) and 1.312 Å (C–F), and an F–C–F bond angle of 108.0°.
Perchloryl fluoride is a reactive gas with the chemical formula ClO
3F. It has a characteristic sweet odor that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid.

Hexachloroethane (perchloroethane) is an organochlorine compound with the chemical formula (CCl3)2. It is a white or colorless solid at room temperature with a camphor-like odor. It has been used by the military in smoke compositions, such as base-eject smoke munitions.

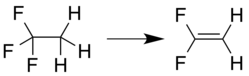
Vinyl fluoride is an organic halide with the chemical formula C2H3F. It is a colorless gas with a faint ether-like odor. It is used as the monomeric precursor to the fluoropolymer polyvinylfluoride.
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula Cl2FC−CClF2. This colorless, volatile liquid is a versatile solvent.