Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.
1-Hexanol (IUPAC name hexan-1-ol) is an organic alcohol with a six-carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Two additional straight chain isomers of 1-hexanol, 2-hexanol and 3-hexanol, exist, both of which differing by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry.

Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.
In organic chemistry, dihydroxybenzenes (benzenediols) are organic compounds in which two hydroxyl groups are substituted onto a benzene ring. These aromatic compounds are classed as phenols. There are three structural isomers: 1,2-dihydroxybenzene is commonly known as catechol, 1,3-dihydroxybenzene is commonly known as resorcinol, and 1,4-dihydroxybenzene is commonly known as hydroquinone.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
In organic chemistry a halohydrin is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups. The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups at various positions relative to the hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl groups in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself.

Oxazines are heterocyclic organic compounds containing one oxygen and one nitrogen atom in a cyclohexa-1,4-diene ring. Isomers exist depending on the relative position of the heteroatoms and relative position of the double bonds.

4-Aminophenol (or para-aminophenol or p-aminophenol) is an organic compound with the formula H2NC6H4OH. Typically available as a white powder, it is commonly used as a developer for black-and-white film, marketed under the name Rodinal.
3-Aminophenol is an organic compound with formula C6H4(NH2)(OH). It is an aromatic amine and a phenol. It is the meta isomer of 2-aminophenol and 4-aminophenol.
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains.

2-Nitrobenzaldehyde is an organic compound with the formula O2NC6H4CHO. It is one of three isomers of nitrobenzaldehyde. It contains a nitro group adjacent to the formyl group.
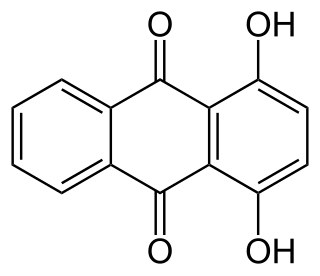
1,4-Dihydroxyanthraquinone, also called quinizarin or Solvent Orange 86, is an organic compound derived from anthroquinone. Quinizarin is an orange or red-brown crystalline powder. It is formally derived from anthraquinone by replacement of two hydrogen atoms by hydroxyl (OH) groups. It is one of ten dihydroxyanthraquinone isomers and occurs in small amounts in the root of the madder plant, Rubia tinctorum.
A tetrahydroxyanthraquinone, also called tetrahydroxyanthradione, is any of several isomeric organic compounds with formula (C12H44)(CO)2, almost invariably derived from 9,10-anthraquinone by replacing four hydrogen atoms by hydroxyl groups. Only a few of these isomers are commercially significant. These are 1,2,5,8-tetrahydroxyanthraquinone (quinalizarin), 1,4,5,8-tetrahydroxyanthraquinone, and 1,2,3,4-tetrahydroxyanthraquinone.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.
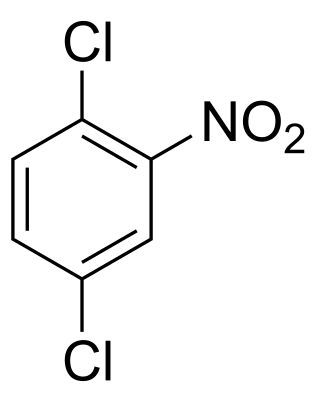
1,4-Dichloro-2-nitrobenzene is an organic compound with the formula C6H3Cl2NO2. One of several isomers of dichloronitrobenzene, it is a yellow solid that is insoluble in water. It is produced by nitration of 1,4-dichlorobenzene. It is a precursor to many derivatives of commercial interest. Hydrogenation gives 1,4-dichloroaniline. Nucleophiles displace the chloride adjacent to the nitro group: ammonia gives the aniline derivative, aqueous base gives the phenol derivative, and methoxide gives the anisole derivative. These compounds are respectively 4-chloro-2-nitroaniline, 4-chloro-2-nitrophenol, and 4-chloro-2-nitroanisole.
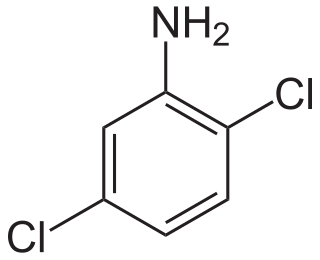
2,5-Dichloroaniline is an organic compound with the formula C6H3Cl2NH2. One of six isomers of dichloroaniline, it is a colorless solid that is insoluble in water. It is produced by hydrogenation of 1,4-dichloro-2-nitrobenzene. It is a precursor to dyes and pigments, e.g., Pigment Yellow 10.