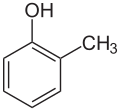
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Methylphenol | |||
| Systematic IUPAC name 2-Methylbenzenol | |||
| Other names 2-Cresol o-Cresol ortho-Cresol ortho-Toluenol ortho-Benzol 2-Hydroxytoluene o-Cresylic acid 1-Hydroxy-2-methylbenzene | |||
| Identifiers | |||
3D model (JSmol) | |||
| 506917 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.204 | ||
| EC Number |
| ||
| 101619 | |||
| KEGG | |||
| MeSH | 2-Cresol | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2076, 3455 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C7H8O | |||
| Molar mass | 108.140 g·mol−1 | ||
| Appearance | Colorless to white crystals | ||
| Odor | sweet, phenolic odor | ||
| Density | 1.0465 g cm−3 | ||
| Melting point | 31 °C; 88 °F; 304 K | ||
| Boiling point | 191 °C; 376 °F; 464 K | ||
| 31 g dm−3 (at 40 °C) | |||
| Solubility | soluble in chloroform, ether, CCl4 | ||
| Solubility in ethanol | Miscible (at 30 °C) | ||
| Solubility in diethyl ether | Miscible (at 30 °C) | ||
| log P | 1.962 | ||
| Vapor pressure | 40 Pa (at 20 °C) | ||
| Acidity (pKa) | 10.316 | ||
| Basicity (pKb) | 3.681 | ||
| −72.9×10−6 cm3/mol | |||
Refractive index (nD) | 1.5353 | ||
| Viscosity | 35.06 cP (at 45 °C) | ||
| Thermochemistry | |||
Heat capacity (C) | 154.56 J K−1 mol−1 | ||
Std molar entropy (S⦵298) | 165.44 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) | −204.3 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) | −3.6936 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H301, H311, H314 | |||
| P260, P264, P270, P280, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P361, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 81 °C (178 °F; 354 K) | ||
| 598.9 °C (1,110.0 °F; 872.0 K) | |||
| Explosive limits | 1.4%–? (148 °C) [1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 1350 mg/kg (rat, oral) 121 mg/kg (rat, oral) 344 mg/kg (mouse, oral) [2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 5 ppm (22 mg/m3) [skin] [1] | ||
REL (Recommended) | TWA 2.3 ppm (10 mg/m3) [1] | ||
IDLH (Immediate danger) | 250 ppm [1] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related phenols | Cresols: | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
ortho-Cresol (IUPAC name: 2-methylphenol, also known as 2-hydroxytoluene or ortho-toluenol) is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and m-cresol. [3]