 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
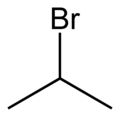
| Preferred IUPAC name 2-Bromopropane [1] | |||
| Other names Isopropyl bromide [2] | |||
| Identifiers | |||
3D model (JSmol) | |||
| 741852 | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.778 | ||
| EC Number |
| ||
| MeSH | 2-bromopropane | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2344 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C3H7Br | |||
| Molar mass | 122.993 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.31 g mL−1 | ||
| Melting point | −89.0 °C; −128.1 °F; 184.2 K | ||
| Boiling point | 59 to 61 °C; 138 to 142 °F; 332 to 334 K | ||
| 3.2 g L−1 (at 20 °C) | |||
| log P | 2.136 | ||
| Vapor pressure | 32 kPa (at 20 °C) | ||
Henry's law constant (kH) | 1.0 μmol Pa−1 mol−1 | ||
Refractive index (nD) | 1.4251 | ||
| Viscosity | 0.4894 mPa s (at 20 °C) | ||
| Thermochemistry | |||
Heat capacity (C) | 135.6 J K mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) | −129 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) | −2.0537–−2.0501 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H225, H360, H373 | |||
| P210, P308+P313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 19 °C (66 °F; 292 K) | ||
| Related compounds | |||
Related alkanes | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
2-Bromopropane, also known as isopropyl bromide and 2-propyl bromide, is the halogenated hydrocarbon with the formula CH3CHBrCH3. It is a colorless liquid. It is used for introducing the isopropyl functional group in organic synthesis. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid. [3]


