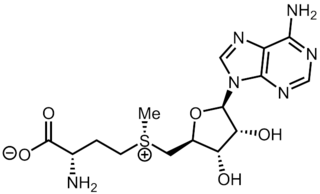
S-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952.

Methionine synthase also known as MS, MeSe, MTR is responsible for the regeneration of methionine from homocysteine. In humans it is encoded by the MTR gene (5-methyltetrahydrofolate-homocysteine methyltransferase). Methionine synthase forms part of the S-adenosylmethionine (SAMe) biosynthesis and regeneration cycle, and is the enzyme responsible for linking the cycle to one-carbon metabolism via the folate cycle. There are two primary forms of this enzyme, the Vitamin B12 (cobalamin)-dependent (MetH) and independent (MetE) forms, although minimal core methionine synthases that do not fit cleanly into either category have also been described in some anaerobic bacteria. The two dominant forms of the enzymes appear to be evolutionary independent and rely on considerably different chemical mechanisms. Mammals and other higher eukaryotes express only the cobalamin-dependent form. In contrast, the distribution of the two forms in Archaeplastida (plants and algae) is more complex. Plants exclusively possess the cobalamin-independent form, while algae have either one of the two, depending on species. Many different microorganisms express both the cobalamin-dependent and cobalamin-independent forms.

Spermidine synthase is an enzyme that catalyzes the transfer of the propylamine group from S-adenosylmethioninamine to putrescine in the biosynthesis of spermidine. The systematic name is S-adenosyl 3-(methylthio)propylamine:putrescine 3-aminopropyltransferase and it belongs to the group of aminopropyl transferases. It does not need any cofactors. Most spermidine synthases exist in solution as dimers.

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leaving group and the methyl group attached to it acts as the electrophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases. Another type of methyl transfer is the radical S-Adenosyl methionine (SAM) which is the methylation of unactivated carbon atoms in primary metabolites, proteins, lipids, and RNA.
In enzymology, a 3'-demethylstaurosporine O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an anthranilate N-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a cyclopropane-fatty-acyl-phospholipid synthase is an enzyme that catalyzes the chemical reaction
In enzymology, an isoflavone 7-O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a putrescine N-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-scoulerine 9-O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a sterol 24-C-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a tabersonine 16-O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a theobromine synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a tRNA (uracil-5-)-methyltransferase is an enzyme that catalyzes the chemical reaction

[Methionine synthase] reductase, or Methionine synthase reductase, encoded by the gene MTRR, is an enzyme that is responsible for the reduction of methionine synthase inside human body. This enzyme is crucial for maintaining the one carbon metabolism, specifically the folate cycle. The enzyme employs one coenzyme, flavoprotein.

Lipoyl synthase is an enzyme that belongs to the radical SAM (S-adenosyl methionine) family. Within the radical SAM superfamily, lipoyl synthase is in a sub-family of enzymes that catalyze sulfur insertion reactions. The enzymes in this subfamily differ from general radical SAM enzymes, as they contain two 4Fe-4S clusters. From these clusters, the enzymes obtain the sulfur groups that will be transferred onto the corresponding substrates. This particular enzyme participates in the final step of lipoic acid metabolism, transferring two sulfur atoms from its 4Fe-4S cluster onto the protein N6-(octanoyl)lysine through radical generation. This enzyme is usually localized to the mitochondria. Two organisms that have been extensively studied with regards to this enzyme are Escherichia coli and Mycobacterium tuberculosis. It is currently being studied in other organisms including yeast, plants, and humans.
Caffeine synthase is a methyltransferase enzyme involved in the caffeine biosynthesis pathway. It is expressed in tea species, coffee species, and cocoa species. The enzyme catalyses the following reactions:
Radical SAM is a designation for a superfamily of enzymes that use a [4Fe-4S]+ cluster to reductively cleave S-adenosyl-L-methionine (SAM) to generate a radical, usually a 5′-deoxyadenosyl radical (5'-dAdo), as a critical intermediate. These enzymes utilize this radical intermediate to perform diverse transformations, often to functionalize unactivated C-H bonds. Radical SAM enzymes are involved in cofactor biosynthesis, enzyme activation, peptide modification, post-transcriptional and post-translational modifications, metalloprotein cluster formation, tRNA modification, lipid metabolism, biosynthesis of antibiotics and natural products etc. The vast majority of known radical SAM enzymes belong to the radical SAM superfamily, and have a cysteine-rich motif that matches or resembles CxxxCxxC. rSAMs comprise the largest superfamily of metal-containing enzymes.
2,7,4'-Trihydroxyisoflavanone 4'-O-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:2,7,4'-trihydroxyisoflavanone 4'-O-methyltransferase . This enzyme catalyses the following chemical reaction
Geranyl diphosphate 2-C-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:geranyl-diphosphate 2-C-methyltransferase. This enzyme catalyses the following chemical reaction






