
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Indigo dye is an organic compound with a distinctive blue color. Historically, indigo was a natural dye extracted from the leaves of some plants of the Indigofera genus, in particular Indigofera tinctoria; dye-bearing Indigofera plants were commonly grown and used throughout the world, in Asia in particular, as an important crop, with the production of indigo dyestuff economically important due to the previous rarity of some blue dyestuffs historically.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.

Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.

Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the number of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink, it is also used as a hydration indicator for silica gel. Methyl violet 10B is also known as crystal violet and has medical uses.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.
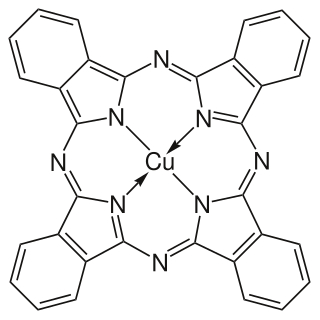
Copper phthalocyanine (CuPc), also called phthalocyanine blue, phthalo blue and many other names, is a bright, crystalline, synthetic blue pigment from the group of phthalocyanine dyes. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalis and acids. It has the appearance of a blue powder, insoluble in most solvents including water.

Resorcinol (or resorcin) is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or meta-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide.

Acid dyes are anionic, soluble in water and are essentially applied from acidic bath. These dyes possess acidic groups, such as SO3H and COOH and are applied on wool, silk and nylon when ionic bond is established between protonated –NH2 group of fibre and acid group of dye. Overall wash fastness is poor although lightfastness is quite good. As dye and fibre contain opposite electrical nature, strike rate and uptake of acid dye on these fibres is faster; electrolyte at higher concentration is added to retard dye uptake and to form levelled shades. Acid generates cation on fibre and temperature helps to substitute negative part of acid with anionic dye molecules.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a natural product of the coumarin family.

Benzidine (trivial name), also called 1,1'-biphenyl-4,4'-diamine (systematic name), is an organic compound with the formula (C6H4NH2)2. It is an aromatic amine. It is a component of a test for cyanide. Related derivatives are used in the production of dyes. Benzidine has been linked to bladder and pancreatic cancer.

Litmus is a water-soluble mixture of different dyes extracted from lichens. It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity.

Alcian blue is any member of a family of polyvalent basic dyes, of which the Alcian blue 8G has been historically the most common and the most reliable member. It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used cationic dyes for both light and electron microscopy. Use of alcian blue has historically been a popular staining method in histology especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called "Alcianophilic". Alcian blue staining can be combined with H&E staining, PAS staining and van Gieson staining methods. Alcian blue can be used to quantitate acidic glycans both in microspectrophotometric quantitation in solution or for staining glycoproteins in polyacrylamide gels or on western blots. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue method.
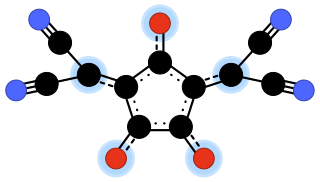
Croconate violet or 1,3-bis(dicyanomethylene)croconate is a divalent anion with chemical formula C
11N
4O2−
3 or ((N≡C−)2C=)2(C5O3)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of two oxygen atoms by dicyanomethylene groups =C(−C≡N)2. Its systematic name is 3,5-bis(dicyanomethylene)-1,2,4-trionate. The term croconate violet as a dye name specifically refers to the dipotassium salt K
2C
11N
4O
3.

Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however it is not commercially available unlike iron(III) nitrate due to its instability to air. The salt is soluble in water serves as a ready source of ferrous ions.






















