
Gene therapy is a medical technology that aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells.

Lipoprotein lipase (LPL) (EC 3.1.1.34, systematic name triacylglycerol acylhydrolase (lipoprotein-dependent)) is a member of the lipase gene family, which includes pancreatic lipase, hepatic lipase, and endothelial lipase. It is a water-soluble enzyme that hydrolyzes triglycerides in lipoproteins, such as those found in chylomicrons and very low-density lipoproteins (VLDL), into two free fatty acids and one monoacylglycerol molecule:
Virotherapy is a treatment using biotechnology to convert viruses into therapeutic agents by reprogramming viruses to treat diseases. There are three main branches of virotherapy: anti-cancer oncolytic viruses, viral vectors for gene therapy and viral immunotherapy. These branches use three different types of treatment methods: gene overexpression, gene knockout, and suicide gene delivery. Gene overexpression adds genetic sequences that compensate for low to zero levels of needed gene expression. Gene knockout uses RNA methods to silence or reduce expression of disease-causing genes. Suicide gene delivery introduces genetic sequences that induce an apoptotic response in cells, usually to kill cancerous growths. In a slightly different context, virotherapy can also refer more broadly to the use of viruses to treat certain medical conditions by killing pathogens.

Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. They are small replication-defective, nonenveloped viruses and have linear single-stranded DNA (ssDNA) genome of approximately 4.8 kilobases (kb).
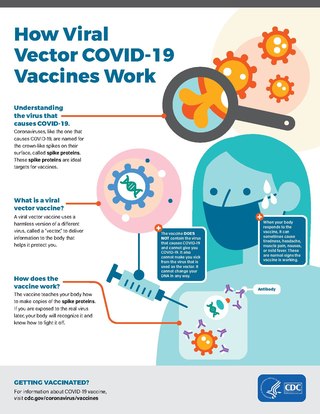
Viral vectors are modified viruses designed to deliver genetic material into cells. This process can be performed inside an organism or in cell culture. Viral vectors have widespread applications in basic research, agriculture, and medicine.
A helper dependent virus, also termed a gutless virus, is a synthetic viral vector dependent on the assistance of a helper virus in order to replicate, and can be used for purposes such as gene therapy. Naturally-occurring satellite viruses are also helper virus dependent, and can sometimes be modified to become viral vectors.

Lipoprotein lipase deficiency is a genetic disorder in which a person has a defective gene for lipoprotein lipase, which leads to very high triglycerides, which in turn causes stomach pain and deposits of fat under the skin, and which can lead to problems with the pancreas and liver, which in turn can lead to diabetes. The disorder only occurs if a child acquires the defective gene from both parents. It is managed by restricting fat in diet to less than 20 g/day.
In biochemistry, lipase refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms.
Retinal gene therapy holds a promise in treating different forms of non-inherited and inherited blindness.

Lysosomal acid lipase deficiency or Wolman Disease, is an autosomal recessive inborn error of metabolism that results in the body not producing enough active lysosomal acid lipase (LAL) enzyme. This enzyme plays an important role in breaking down fatty material in the body. Infants, children and adults that have LAL deficiency experience a range of serious health problems. The lack of the LAL enzyme can lead to a build-up of fatty material in a number of body organs including the liver, spleen, gut, in the wall of blood vessels and other important organs.
Mydicar is a genetically targeted enzyme replacement therapy being studied for use in patients with severe heart failure. It is designed to increase the level of SERCA2a, a sarcoplasmic endoplasmic reticulum calcium (Ca2+) ATPase found in the membrane of the sarcoplasmic reticulum (SR). The SERCA2a gene is delivered to the heart via an adeno-associated viral vector. Using the α-myosin heavy chain gene promoter in the cardiac muscle cells, also called cardiomyocytes, Mydicar is able to direct the gene expression only to the heart muscle. Mydicar is being tested in a phase 2 study, in which has been compared to a placebo in 39 advanced heart failure patients. Thus far, patients treated with Mydicar have shown a 52% reduction in the risk of worsening heart failure compared to patients treated with the placebo.
Gene therapy for osteoarthritis is the application of gene therapy to treat osteoarthritis (OA). Unlike pharmacological treatments which are administered locally or systemically as a series of interventions, gene therapy aims to establish sustained therapeutic effect after a single, local injection.
Self-complementary adeno-associated virus (scAAV) is a viral vector engineered from the naturally occurring adeno-associated virus (AAV) to be used as a tool for gene therapy. Use of recombinant AAV (rAAV) has been successful in clinical trials addressing a variety of diseases. This lab-made progeny of rAAV is termed "self-complementary" because the coding region has been designed to form an intra-molecular double-stranded DNA template. A rate-limiting step for the standard AAV genome involves the second-strand synthesis since the typical AAV genome is a single-stranded DNA template. However, this is not the case for scAAV genomes. Upon infection, rather than waiting for cell mediated synthesis of the second strand, the two complementary halves of scAAV will associate to form one double stranded DNA (dsDNA) unit that is ready for immediate replication and transcription. The caveat of this construct is that instead of the full coding capacity found in rAAV (4.7–6kb) scAAV can only hold about half of that amount (≈2.4kb).

Glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 (GPI-HBP1) also known as high density lipoprotein-binding protein 1 is a protein that in humans is encoded by the GPIHBP1 gene.
James M. Wilson is an American biomedical researcher and CEO of two biotech companies, Gemma Biotherapeutics and Franklin Biolabs, focused on gene therapies. He previously served as the Director of the Gene Therapy Program, Rose H. Weiss Professor and Director of the Orphan Disease Center, and Professor of Medicine and Pediatrics at the Perelman School of Medicine at the University of Pennsylvania. Previously, he held the John Herr Musser endowed professorship at the Perelman School of Medicine.
Adeno-associated virus (AAV) has been researched as a viral vector in gene therapy for cancer treatment as an oncolytic virus. Currently there are not any FDA approved AAV cancer treatments, as the first FDA approved AAV treatment was approved December 2017. However, there are many Oncolytic AAV applications that are in development and have been researched.
Richard Jude Samulski is an American scientist, inventor, and academic recognized for his pioneering work in gene therapy and adeno-associated virus vectors (AAV) in the fields of molecular virology and pharmacology.
Jean Bennett is the F. M. Kirby Professor of Ophthalmology in the Perelman School of Medicine at the University of Pennsylvania. Her research focuses on gene therapy for retinal diseases. Her laboratory developed the first FDA approved gene therapy for use in humans, which treats a rare form of blindness. She was elected a member of the National Academy of Sciences in 2022.
Valoctocogene roxaparvovec, sold under the brand name Roctavian, is a gene therapy used for the treatment of hemophilia A. It was developed by BioMarin Pharmaceutical. Valoctocogene roxaparvovec is made of a virus (AAV5) that has been modified to contain the gene for factor VIII, which is lacking in people with hemophilia A. It is an adeno-associated virus vector-based gene therapy. It is given by intravenous infusion.







