Related Research Articles

An insulin pump is a medical device used for the administration of insulin in the treatment of diabetes mellitus, also known as continuous subcutaneous insulin therapy. The device configuration may vary depending on design. A traditional pump includes:

Blood glucose monitoring is the use of a glucose meter for testing the concentration of glucose in the blood (glycemia). Particularly important in diabetes management, a blood glucose test is typically performed by piercing the skin to draw blood, then applying the blood to a chemically active disposable 'test-strip'. The other main option is continuous glucose monitoring (CGM). Different manufacturers use different technology, but most systems measure an electrical characteristic and use this to determine the glucose level in the blood. Skin-prick methods measure capillary blood glucose, whereas CGM correlates interstitial fluid glucose level to blood glucose level. Measurements may occur after fasting or at random nonfasting intervals, each of which informs diagnosis or monitoring in different ways.
Glycated hemoglobin, glycohemoglobin, glycosylated hemoglobin is a form of hemoglobin (Hb) that is chemically linked to a sugar. Several types of glycated hemoglobin measures exist, of which HbA1c, or simply A1c, is a standard single test. Most monosaccharides, including glucose, galactose, and fructose, spontaneously bond with hemoglobin when present in the bloodstream. However, glucose is only 21% as likely to do so as galactose and 13% as likely to do so as fructose, which may explain why glucose is used as the primary metabolic fuel in humans.

A glucose meter, also referred to as a "glucometer", is a medical device for determining the approximate concentration of glucose in the blood. It can also be a strip of glucose paper dipped into a substance and measured to the glucose chart. It is a key element of glucose testing, including home blood glucose monitoring (HBGM) performed by people with diabetes mellitus or hypoglycemia. A small drop of blood, obtained from slightly piercing a fingertip with a lancet, is placed on a disposable test strip that the meter reads and uses to calculate the blood glucose level. The meter then displays the level in units of mg/dL or mmol/L.
The term diabetes includes several different metabolic disorders that all, if left untreated, result in abnormally high concentrations of a sugar called glucose in the blood. Diabetes mellitus type 1 results when the pancreas no longer produces significant amounts of the hormone insulin, usually owing to the autoimmune destruction of the insulin-producing beta cells of the pancreas. Diabetes mellitus type 2, in contrast, is now thought to result from autoimmune attacks on the pancreas and/or insulin resistance. The pancreas of a person with type 2 diabetes may be producing normal or even abnormally large amounts of insulin. Other forms of diabetes mellitus, such as the various forms of maturity-onset diabetes of the young, may represent some combination of insufficient insulin production and insulin resistance. Some degree of insulin resistance may also be present in a person with type 1 diabetes.

Inhalable insulin is a powdered form of insulin, delivered with an inhaler into the lungs where it is absorbed. In general inhaled insulins have been more rapidly absorbed than subcutaneous injected insulin, with faster peak concentration in serum and more rapid metabolism.
Automated insulin delivery systems are automated systems designed to assist people with insulin-requiring diabetes, by automatically adjusting insulin delivery in response to blood glucose levels. Currently available systems can only deliver a single hormone—insulin. Other systems currently in development aim to improve on current systems by adding one or more additional hormones that can be delivered as needed, providing something closer to the endocrine functionality of the pancreas.
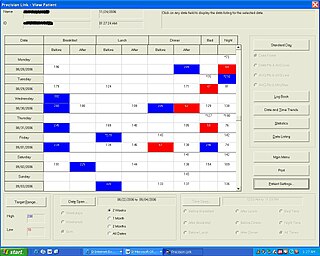
Diabetes Management Software refers to software tools that run on personal computers and personal digital assistants to help persons with Type 1 and Type 2 diabetes manage the data associated with:
Noninvasive glucose monitoring (NIGM), called Noninvasive continuous glucose monitoring when used as a CGM technique, is the measurement of blood glucose levels, required by people with diabetes to prevent both chronic and acute complications from the disease, without drawing blood, puncturing the skin, or causing pain or trauma. The search for a successful technique began about 1975 and has continued to the present without a clinically or commercially viable product.
Prediabetes is a component of metabolic syndrome and is characterized by elevated blood sugar levels that fall below the threshold to diagnose diabetes mellitus. It usually does not cause symptoms but people with prediabetes often have obesity, dyslipidemia with high triglycerides and/or low HDL cholesterol, and hypertension. It is also associated with increased risk for cardiovascular disease (CVD). Prediabetes is more accurately considered an early stage of diabetes as health complications associated with type 2 diabetes often occur before the diagnosis of diabetes.
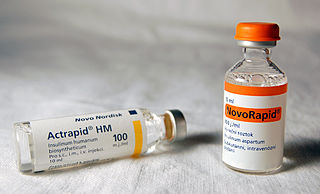
As a medication, insulin is any pharmaceutical preparation of the protein hormone insulin that is used to treat high blood glucose. Such conditions include type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. Insulin is also used along with glucose to treat hyperkalemia. Typically it is given by injection under the skin, but some forms may also be used by injection into a vein or muscle. There are various types of insulin, suitable for various time spans. The types are often all called insulin in the broad sense, although in a more precise sense, insulin is identical to the naturally occurring molecule whereas insulin analogues have slightly different molecules that allow for modified time of action. It is on the World Health Organization's List of Essential Medicines. In 2021, it was the 179th most commonly prescribed medication in the United States, with more than 2 million prescriptions.

MiniMed Paradigm is a series of insulin pumps manufactured by Medtronic for patients with diabetes mellitus. The pump operates with a single AAA battery and uses a piston-plunger pump to infuse a programmed amount of insulin into the patient through a length of tubing. The Paradigm uses a one-way wireless radio frequency link to receive blood sugar measurements from select glucose meters. The Paradigm RT series adds the ability to receive data from a mated continuous blood-glucose monitor. Although the pump can use these measurements to assist in calculating a dose of insulin, no actual change in insulin delivery occurs without manual user-intervention.
DexCom, Inc. is a company that develops, manufactures, produces, and distributes continuous glucose monitoring (CGM) systems for diabetes management. It operates internationally with headquarters in San Diego, California, and has manufacturing facilities in Mesa, Arizona and Batu Kawan, Malaysia.
Bruce Bode, MD, FACE is a diabetes specialist with the Atlanta Diabetes Associates in Atlanta, GA and is a clinical associate professor at Emory University in the Department of Medicine. He has served on the board of directors of the Atlanta chapters of the Juvenile Diabetes Research Foundation (JDRF), the American Diabetes Association (ADA), and various Georgia-based diabetes camps. Bode is a member of the board of directors of Glytec and an active member of the JDRF research team validating the efficacy and safety of real-time continuous glucose monitoring (CGMS), and is a former president of the ADA Georgia Affiliate and editor of the ADA's 2004 edition of Medical Management of Type 1 Diabetes.
International Diabetes Center at Park Nicollet (IDC) is a center for diabetes care, research and education located in Minneapolis, Minnesota, United States. The center provides clinical, motivational and educational services for people with diabetes. It is part of HealthPartners Institute.
Tandem Diabetes Care, Inc. is an American medical device manufacturer based in San Diego, California. The company develops medical technologies for the treatment of diabetes and specifically insulin infusion therapy.
The Open Artificial Pancreas System (OpenAPS) project is a free and open-source project that aims to make basic artificial pancreas system (APS) technology available to everyone. The OpenAPS project was designed with the idea of quickly getting the APS technology to more people using a direct approach, rather than waiting for clinical trials to be completed and regulatory approval to be granted.

A continuous glucose monitor (CGM) is a device used for monitoring blood glucose on a continual basis instead of monitoring glucose levels periodically by drawing a drop of blood from a finger. This is known as continuous glucose monitoring. CGMs are used by people who treat their diabetes with insulin, for example people with type 1 diabetes, type 2 diabetes, or other types of diabetes, such as gestational diabetes.
William V. Tamborlane has been Professor and Chief of Pediatric Endocrinology at Yale School of Medicine since 1986.
Anne Peters is a endocrinologist, diabetes expert, and professor of clinical medicine at the Keck School of Medicine of USC. She runs diabetes centers in well-served Beverly Hills and under-resourced East Los Angeles. She teaches physicians and people with diabetes around the world how to better treat the condition, through lifestyle, medications and technology.
References
- ↑ Mazze, Roger S; Lucido, David; Langer, Oded; Hartmann, Klaus; Rodbard, David (1987-01-01). "Ambulatory Glucose Profile: Representation of Verified Self-Monitored Blood Glucose Data". Diabetes Care. 10 (1). American Diabetes Association: 111–117. doi:10.2337/diacare.10.1.111. ISSN 0149-5992.
- 1 2 3 4 Danne, Thomas; Nimri, Revital; Battelino, Tadej; Bergenstal, Richard M.; Close, Kelly L.; DeVries, J. Hans; Garg, Satish; Heinemann, Lutz; Hirsch, Irl; Amiel, Stephanie A.; Beck, Roy; Bosi, Emanuele; Buckingham, Bruce; Cobelli, Claudio; Dassau, Eyal; Doyle, Francis J.; Heller, Simon; Hovorka, Roman; Jia, Weiping; Jones, Tim; Kordonouri, Olga; Kovatchev, Boris; Kowalski, Aaron; Laffel, Lori; Maahs, David; Murphy, Helen R.; Nørgaard, Kirsten; Parkin, Christopher G.; Renard, Eric; Saboo, Banshi; Scharf, Mauro; Tamborlane, William V.; Weinzimer, Stuart A.; Phillip, Moshe (2017-11-10). "International Consensus on Use of Continuous Glucose Monitoring". Diabetes Care. 40 (12). American Diabetes Association: 1631–1640. doi:10.2337/dc17-1600. ISSN 0149-5992.
- ↑ Mazze R. Langer O. Innovative Technologies in Research and Care in Diabetes and Pregnancy. Israel J of Medical Science, 1993.
- ↑ Langer, Oded; Mazze, Roger (1988). "The relationship between large-for-gestational-age infants and glycemic control in women with gestational diabetes". American Journal of Obstetrics and Gynecology. 159 (6). Elsevier BV: 1478–1483. doi:10.1016/0002-9378(88)90578-9. ISSN 0002-9378.
- ↑ Mazze R, Langer O. Assessing Metabolic Control and Improving Patient Management: The Application of Computer Technology. Diabetes Research and Clinical Practice, October 1990.
- ↑ Mazze R, Strock E, Wesley D, Borgman S, Morgan B, Bergenstal R, Cuddihy R. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile (AGP) analysis. Diabetes Technology and Therapeutics, 2008; 10(3).
- ↑ Mazze R, Strock E, Borgman S, Wesley D, Stout P, Racchini J. Evaluating the Accuracy, Reliability, and Clinical Applicability of Continuous Glucose Monitoring (CGM): Is CGM Ready for Real Time? Diabetes Technology & Therapeutics, 2009;11(1).
- ↑ Mazze R, Strock E, Morgan B, Wesley D, Cuddihy R, Bergenstal R. Diurnal glucose patterns of Exenatide Once Weekly: A 1 year study using continuous glucose monitoring and Ambulatory Glucose Profile analysis. Endocrine Practice 2009;15(4):326-34.
- ↑ Bolli G, Deeb L, Garg S, Leahy J, Mazze R, Owens D, Riddle M, Southerland P, Strock E. International Forum for the Advancement of Diabetes Research and Care, April 29–30, 2011, Athens, Greece. Diabetes Technology & Therapeutics 2011;13(9):967-979.
- ↑ Mazze R, Yogev Y, Langer O. Measuring glucose exposure and variability using continuous glucose monitoring in normal and abnormal glucose metabolism in pregnancy. Journal of Maternal-Fetal and Neonatal Medicine 2012, vol./is. 25/7(1171-5).
- 1 2 Bergenstal R, Ahman AJ, Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technology and Therapeutics,2013;15(3): 198-211.
- ↑ Matthaei S, Antuna Dealaiz R, Bosi, E, et al. Consensus recommendations for the use of Ambulatory Glucose Profile in clinical practice. British Journal of Diabetes and Vascular Disease 2014, 14:153-157.
- 1 2 3 4 Petrie, John R.; Peters, Anne L.; Bergenstal, Richard M.; Holl, Reinhard W.; Fleming, G. Alexander; Heinemann, Lutz (1 December 2017). "Improving the Clinical Value and Utility of CGM Systems: Issues and Recommendations". Diabetes Care . 40 (12): 1614–1621. doi:10.2337/dci17-0043.
- 1 2 Riddle, M. C., Gerstein, H. C., & Cefalu, W. T. (2017). Maturation of CGM and Glycemic Measurements Beyond HbA1c—A Turning Point in Research and Clinical Decisions. Diabetes Care, 40(12), 1611-1613.
- ↑ Hoss U, Budiman E, Liu H Christiansen H. Continuous Glucose Monitoring in the Subcutaneous Tissue over a 14-Day Sensor Wear Period Diabetes. Sci Technol. Sep 2013; 7(5): 1210–1219.
- ↑ Buckingham BA, Close KL, Bergenstal RM, Danne T, Grunberger G, Kowalski AJ, Peters A, Heller SR. Reaching an International Consensus on Standardizing Continuous Glucose Monitoring (CGM) Outcomes―Aligning Clinicians, Researchers, Patients, and Regulators. American Diabetes Association 77th Scientific Meeting, San Diego, CA, June 2017.
- 1 2 3 "Welcome to AGP Report | AGP Report".