
Neomycin is an aminoglycoside antibiotic that displays bactericidal activity against gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against gram-positive bacilli and anaerobic gram-negative bacilli. Neomycin comes in oral and topical formulations, including creams, ointments, and eyedrops. Neomycin belongs to the aminoglycoside class of antibiotics that contain two or more amino sugars connected by glycosidic bonds.

Kanamycin A, often referred to simply as kanamycin, is an antibiotic used to treat severe bacterial infections and tuberculosis. It is not a first line treatment. It is used by mouth, injection into a vein, or injection into a muscle. Kanamycin is recommended for short-term use only, usually from 7 to 10 days. As with most antibiotics, it is ineffective in viral infections.

Amikacin is an antibiotic medication used for a number of bacterial infections. This includes joint infections, intra-abdominal infections, meningitis, pneumonia, sepsis, and urinary tract infections. It is also used for the treatment of multidrug-resistant tuberculosis. It is used by injection into a vein using an IV or into a muscle.

N-acetyltransferase (NAT) is an enzyme that catalyzes the transfer of acetyl groups from acetyl-CoA to arylamines, arylhydroxylamines and arylhydrazines. They have wide specificity for aromatic amines, particularly serotonin, and can also catalyze acetyl transfer between arylamines without CoA. N-acetyltransferases are cytosolic enzymes found in the liver and many tissues of most mammalian species, except the dog and fox, which cannot acetylate xenobiotics.

Aralkylamine N-acetyltransferase (AANAT), also known as arylalkylamine N-acetyltransferase or serotonin N-acetyltransferase (SNAT), is an enzyme that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb AANAT gene containing four exons, located on chromosome 17q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80 percent identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of N-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function.
In enzymology, an alpha-tubulin N-acetyltransferase is an enzyme which is encoded by the ATAT1 gene.
In enzymology, an aminoglycoside N3'-acetyltransferase (EC 2.3.1.81) is an enzyme that catalyzes the chemical reaction
In enzymology, an arylamine N-acetyltransferase is an enzyme that catalyzes the chemical reaction

Carnitine O-acetyltransferase also called carnitine acetyltransferase is an enzyme that encoded by the CRAT gene that catalyzes the chemical reaction
In enzymology, a gentamicin 2'-N-acetyltransferase (EC 2.3.1.59) is an enzyme that catalyzes the chemical reaction
In enzymology, a gentamicin 3'-N-acetyltransferase (EC 2.3.1.60) is an enzyme that catalyzes the chemical reaction

In enzymology, glucosamine-phosphate N-acetyltransferase (GNA) is an enzyme that catalyzes the transfer of an acetyl group from acetyl-CoA to the primary amine in glucosamide-6-phosphate, generating a free CoA and N-acetyl-D-glucosamine-6-phosphate.

In enzymology, a homocitrate synthase (EC 2.3.3.14) is an enzyme that catalyzes the chemical reaction

In enzymology, a lysine N-acetyltransferase (EC 2.3.1.32) is an enzyme that catalyzes the chemical reaction
In enzymology, a N6-hydroxylysine O-acetyltransferase (EC 2.3.1.102) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetylneuraminate 4-O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetylneuraminate 7-O(or 9-O)-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a peptide alpha-N-acetyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a serine O-acetyltransferase is an enzyme that catalyzes the chemical reaction
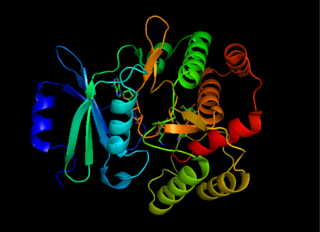
Aminoglycoside-3'-phosphotransferase, also known as aminoglycoside kinase, is an enzyme that primarily catalyzes the addition of phosphate from ATP to the 3'-hydroxyl group of a 4,6-disubstituted aminoglycoside, such as kanamycin. However, APH(3') has also been found to phosphorylate at the 5'-hydroxyl group in 4,5-disubstituted aminoglycosides, which lack a 3'-hydroxyl group, and to diphosphorylate hydroxyl groups in aminoglycosides that have both 3'- and 5'-hydroxyl groups. Primarily positively charged at biological conditions, aminoglycosides bind to the negatively charged backbone of nucleic acids to disrupt protein synthesis, effectively inhibiting bacterial cell growth. APH(3') mediated phosphorylation of aminoglycosides effectively disrupts their mechanism of action, introducing a phosphate group that reduces their binding affinity due to steric hindrances and unfavorable electrostatic interactions. APH(3') is primarily found in certain species of gram-positive bacteria.











