Related Research Articles

A dental implant is a prosthesis that interfaces with the bone of the jaw or skull to support a dental prosthesis such as a crown, bridge, denture, or facial prosthesis or to act as an orthodontic anchor. The basis for modern dental implants is a biological process called osseointegration, in which materials such as titanium or zirconia form an intimate bond to the bone. The implant fixture is first placed so that it is likely to osseointegrate, then a dental prosthetic is added. A variable amount of healing time is required for osseointegration before either the dental prosthetic is attached to the implant or an abutment is placed which will hold a dental prosthetic or crown.

Dental alveoli are sockets in the jaws in which the roots of teeth are held in the alveolar process with the periodontal ligament. The lay term for dental alveoli is tooth sockets. A joint that connects the roots of the teeth and the alveolus is called a gomphosis. Alveolar bone is the bone that surrounds the roots of the teeth forming bone sockets.
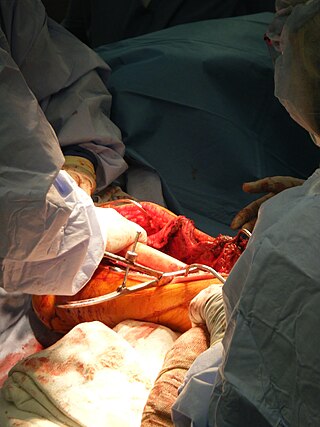
Bone grafting is a surgical procedure that replaces missing bone in order to repair bone fractures that are extremely complex, pose a significant health risk to the patient, or fail to heal properly. Some small or acute fractures can be cured without bone grafting, but the risk is greater for large fractures like compound fractures.

The alveolar process is the portion of bone containing the tooth sockets on the jaw bones. The alveolar process is covered by gums within the mouth, terminating roughly along the line of the mandibular canal. Partially comprising compact bone, it is penetrated by many small openings for blood vessels and connective fibres.
Articular cartilage, most notably that which is found in the knee joint, is generally characterized by very low friction, high wear resistance, and poor regenerative qualities. It is responsible for much of the compressive resistance and load bearing qualities of the knee joint and, without it, walking is painful to impossible. Osteoarthritis is a common condition of cartilage failure that can lead to limited range of motion, bone damage and invariably, pain. Due to a combination of acute stress and chronic fatigue, osteoarthritis directly manifests itself in a wearing away of the articular surface and, in extreme cases, bone can be exposed in the joint. Some additional examples of cartilage failure mechanisms include cellular matrix linkage rupture, chondrocyte protein synthesis inhibition, and chondrocyte apoptosis. There are several different repair options available for cartilage damage or failure.

Maxillary sinus floor augmentation is a surgical procedure which aims to increase the amount of bone in the posterior maxilla, in the area of the premolar and molar teeth, by lifting the lower Schneiderian membrane and placing a bone graft.
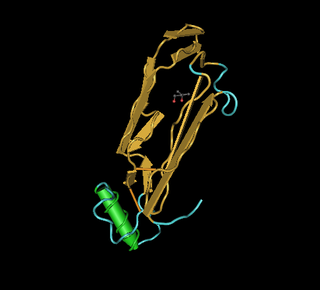
Bone morphogenetic protein 2 or BMP-2 belongs to the TGF-β superfamily of proteins.
“Lateral periodontal cysts (LPCs) are defined as non-keratinised and non-inflammatory developmental cysts located adjacent or lateral to the root of a vital tooth.” LPCs are a rare form of jaw cysts, with the same histopathological characteristics as gingival cysts of adults (GCA). Hence LPCs are regarded as the intraosseous form of the extraosseous GCA. They are commonly found along the lateral periodontium or within the bone between the roots of vital teeth, around mandibular canines and premolars. Standish and Shafer reported the first well-documented case of LPCs in 1958, followed by Holder and Kunkel in the same year although it was called a periodontal cyst. Since then, there has been more than 270 well-documented cases of LPCs in literature.
Guided bone regeneration (GBR) and guided tissue regeneration (GTR) are dental surgical procedures that use barrier membranes to direct the growth of new bone and gingival tissue at sites with insufficient volumes or dimensions of bone or gingiva for proper function, esthetics or prosthetic restoration. Guided bone regeneration typically refers to ridge augmentation or bone regenerative procedures; guided tissue regeneration typically refers to regeneration of periodontal attachment.
Hessam Nowzari is the Director of the University of Southern California Advanced Periodontics program, since 1995, and is a diplomate of the American Board of Periodontology.

Bioceramics and bioglasses are ceramic materials that are biocompatible. Bioceramics are an important subset of biomaterials. Bioceramics range in biocompatibility from the ceramic oxides, which are inert in the body, to the other extreme of resorbable materials, which are eventually replaced by the body after they have assisted repair. Bioceramics are used in many types of medical procedures. Bioceramics are typically used as rigid materials in surgical implants, though some bioceramics are flexible. The ceramic materials used are not the same as porcelain type ceramic materials. Rather, bioceramics are closely related to either the body's own materials or are extremely durable metal oxides.
A fibrin scaffold is a network of protein that holds together and supports a variety of living tissues. It is produced naturally by the body after injury, but also can be engineered as a tissue substitute to speed healing. The scaffold consists of naturally occurring biomaterials composed of a cross-linked fibrin network and has a broad use in biomedical applications.
Socket preservation or alveolar ridge preservation is a procedure to reduce bone loss after tooth extraction. After tooth extraction, the jaw bone has a natural tendency to become narrow, and lose its original shape because the bone quickly resorbs, resulting in 30–60% loss in bone volume in the first six months. Bone loss, can compromise the ability to place a dental implant, or its aesthetics and functional ability.
Octacalcium phosphate (sometimes referred to as OCP) is a form of calcium phosphate with formula Ca8H2(PO4)6·5H2O. OCP may be a precursor to tooth enamel, dentine, and bones. OCP is a precursor of hydroxyapatite (HA), an inorganic biomineral that is important in bone growth. OCP has garnered lots of attention due to its inherent biocompatibility. While OCP exhibits good properties in terms of bone growth, very stringent synthesis requirements make it difficult for mass productions, but nevertheless has shown promise not only in-vitro, but also in in-vivo clinical case studies.

Peri-implantitis is a destructive inflammatory process affecting the soft and hard tissues surrounding dental implants. The soft tissues become inflamed whereas the alveolar bone, which surrounds the implant for the purposes of retention, is lost over time.
Tissue engineering of oral mucosa combines cells, materials and engineering to produce a three-dimensional reconstruction of oral mucosa. It is meant to simulate the real anatomical structure and function of oral mucosa. Tissue engineered oral mucosa shows promise for clinical use, such as the replacement of soft tissue defects in the oral cavity. These defects can be divided into two major categories: the gingival recessions which are tooth-related defects, and the non tooth-related defects. Non tooth-related defects can be the result of trauma, chronic infection or defects caused by tumor resection or ablation. Common approaches for replacing damaged oral mucosa are the use of autologous grafts and cultured epithelial sheets.
Platelet-rich fibrin (PRF) or leukocyte- and platelet-rich fibrin (L-PRF) is a derivative of PRP where autologous platelets and leukocytes are present in a complex fibrin matrix to accelerate the healing of soft and hard tissue and is used as a tissue-engineering scaffold in oral and maxillofacial surgeries. PRF falls under FDA Product Code KST, labeling it as a blood draw/Hematology product classifying it as 510(k) exempt.
Alloplasty is a surgical procedure performed to substitute and repair defects within the body with the use of synthetic material. It can also be performed in order to bridge wounds. The process of undergoing alloplasty involves the construction of an alloplastic graft through the use of computed tomography (CT), rapid prototyping and "the use of computer-assisted virtual model surgery." Each alloplastic graft is individually constructed and customised according to the patient's defect to address their personal health issue. Alloplasty can be applied in the form of reconstructive surgery. An example where alloplasty is applied in reconstructive surgery is in aiding cranial defects. The insertion and fixation of alloplastic implants can also be applied in cosmetic enhancement and augmentation. Since the inception of alloplasty, it has been proposed that it could be a viable alternative to other forms of transplants. The biocompatibility and customisation of alloplastic implants and grafts provides a method that may be suitable for both minor and major medical cases that may have more limitations in surgical approach. Although there has been evidence that alloplasty is a viable method for repairing and substituting defects, there are disadvantages including suitability of patient bone quality and quantity for long term implant stability, possibility of rejection of the alloplastic implant, injuring surrounding nerves, cost of procedure and long recovery times. Complications can also occur from inadequate engineering of alloplastic implants and grafts, and poor implant fixation to bone. These include infection, inflammatory reactions, the fracture of alloplastic implants and prostheses, loosening of implants or reduced or complete loss of osseointegration.

In periodontology, gingival grafting, also called gum grafting or periodontal plastic surgery, is a generic term for the performance of any of a number of surgical procedures in which the gingiva is grafted. The aim may be to cover exposed root surfaces or merely to augment the band of keratinized tissue.
References
- ↑ Carranza, FA; McLain, PK, Schallhorn, RG: Regenerative Osseous Surgery. In Newman, Takei, Carranza, editors: Carranza's Clinical Periodontology, 9th Edition. Philadelphia: W.B. Saunders Co. 2002. page 809.
- ↑ Eickholz, Peter; Kim, Ti‐Sun & Holle, Rolf (1998). "Regenerative periodontal surgery with non‐resorbable and biodegradable barriers: results after 24 months". Journal of Clinical Periodontology. 25 (8): 666–676. doi:10.1111/j.1600-051x.1998.tb02504.x. PMID 9722272.
- 1 2 3 Greenberg, AM; Prein, Joachim: Craniomaxillofacial reconstructive and corrective bone surgery , Springer 2002 ISBN 0-387-94686-1 page 155-6.
- 1 2 3 4 Miller N, Penaud J, Foliguet B, Membre H, Ambrosini P, Plombas M (December 1996). "Resorption rates of 2 commercially available bioresorbable membranes. A histomorphometric study in a rabbit model". J. Clin. Periodontol. 23 (12): 1051–9. doi:10.1111/j.1600-051X.1996.tb01803.x. PMID 8997647.
- ↑ Gottlow J, Nyman S, Karring T, Lindhe J (September 1984). "New attachment formation as the result of controlled tissue regeneration". J. Clin. Periodontol. 11 (8): 494–503. doi:10.1111/j.1600-051X.1984.tb00901.x. PMID 6384274.
- 1 2 3 4 Juodzbalys G, Raustia AM, Kubilius R (October 2007). "A 5-year follow-up study on one-stage implants inserted concomitantly with localized alveolar ridge augmentation". Journal of Oral Rehabilitation. 34 (10): 781–9. doi:10.1111/j.1365-2842.2006.01679.x. PMID 17824891.
- ↑ Perry R. Klokkevold; Newman, Michael C.; Henry H. Takei (2006). Carranza's Clinical Periodontology. Philadelphia: Saunders. ISBN 1-4160-2400-X.
- 1 2 Duskova M, Leamerova E, Sosna B, Gojis O (November 2006). "Guided tissue regeneration, barrier membranes and reconstruction of the cleft maxillary alveolus". J Craniofac Surg. 17 (6): 1153–60. doi:10.1097/01.scs.0000236435.90097.7b. PMID 17119421.
- 1 2 Wang HL, Boyapati L (March 2006). ""PASS" principles for predictable bone regeneration". Implant Dent. 15 (1): 8–17. doi:10.1097/01.id.0000204762.39826.0f. PMID 16569956.
- ↑ Bunyaratavej P, Wang HL (February 2001). "Collagen membranes: a review" (PDF). J. Periodontol. 72 (2): 215–29. doi:10.1902/jop.2001.72.2.215. hdl: 2027.42/141506 . PMID 11288796.
- ↑ Simion M, Scarano A, Gionso L, Piattelli A (1996). "Guided bone regeneration using resorbable and nonresorbable membranes: a comparative histologic study in humans". Int J Oral Maxillofac Implants. 11 (6): 735–42. PMID 8990634.
- ↑ Simion M, Misitano U, Gionso L, Salvato A (1997). "Treatment of dehiscences and fenestrations around dental implants using resorbable and nonresorbable membranes associated with bone autografts: a comparative clinical study". Int J Oral Maxillofac Implants. 12 (2): 159–67. PMID 9109265.
- ↑ Hämmerle CH, Lang NP (February 2001). "Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials". Clinical Oral Implants Research. 12 (1): 9–18. doi:10.1034/j.1600-0501.2001.012001009.x. PMID 11168266.
- ↑ Stavropoulos F, Dahlin C, Ruskin JD, Johansson C (August 2004). "A comparative study of barrier membranes as graft protectors in the treatment of localized bone defects. An experimental study in a canine model". Clinical Oral Implants Research. 15 (4): 435–42. doi:10.1111/j.1600-0501.2004.01029.x. PMID 15248878.
- ↑ Hämmerle CH, Jung RE, Yaman D, Lang NP (January 2008). "Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases" (PDF). Clinical Oral Implants Research. 19 (1): 19–25. doi:10.1111/j.1600-0501.2007.01407.x. PMID 17956571.
- ↑ Chiapasco M, Zaniboni M, Boisco M (October 2006). "Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants". Clinical Oral Implants Research. 17 Suppl 2: 136–59. doi:10.1111/j.1600-0501.2006.01357.x. PMID 16968389.