Aromatic compounds are organic compounds are "mono- and polycyclic aromatic hydrocarbons". The parent member is benzene. Heteroarenes are closely related by at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom.

Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics and pesticides, explosives and drugs. It has also been used to make bile acids, cholesterol and steroids.
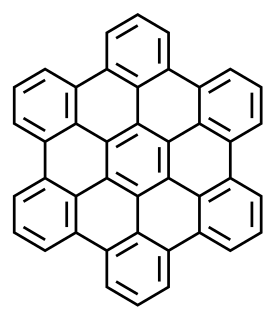
A polycyclic aromatic hydrocarbon (PAH) is a hydrocarbon—a chemical compound containing only carbon and hydrogen—that is composed of multiple aromatic rings. The group is a major subset of the aromatic hydrocarbons. The simplest of such chemicals are naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone, however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

Benzo[a]pyrene is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites (more commonly known as BPDE) react and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.

Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C16H10. This yellow solid is the smallest peri-fused PAH. Pyrene forms during incomplete combustion of organic compounds.

Fluorene, or 9H-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar. It is insoluble in water and soluble in many organic solvents. Although sometimes classified as a polycyclic aromatic hydrocarbon, the five-membered ring has no aromatic properties. Fluorene is mildly acidic.

Anthrone is a tricyclic aromatic ketone. It is used for a common cellulose assay and in the colorimetric determination of carbohydrates.

Solvent Violet 13, also known as D&C Violet No.2, oil violet, Solvent Blue 90, Alizarine Violet 3B, Alizurol Purple, Duranol Brilliant Violet TG, Ahcoquinone Blue IR base, Quinizarin Blue, Disperse Blue 72, and C.I. 60725, is a synthetic anthraquinone dye with bright bluish violet hue. It is a solid insoluble in water and soluble in acetone, toluene, and benzene. Its chemical formula is C21H15NO3, and its structure is 1-hydroxy-4-(p-tolylamino)anthraquinone, or 1-hydroxy-4-[(4-methylphenyl)amino]-9,10-anthracenedione or 1-hydroxy-4-(4-methylanilino)anthraquinone.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5–6 mg/kg.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.

Benz[a]anthracene or benzo[a]anthracene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is produced during incomplete combustion of organic matter.

Benzo[j]fluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo[a]fluoranthene (BaF), bendo[b]fluoranthene (BbF), benzo[e]fluoranthene (BeF), and benzo[k]fluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.

Roland Heinrich Scholl was a Swiss chemist who taught at various European universities. Among his most notable achievements are the synthesis of coronene, the co-development of the Bally-Scholl synthesis, and various discoveries about polycyclic aromatic hydrocarbons.

Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.
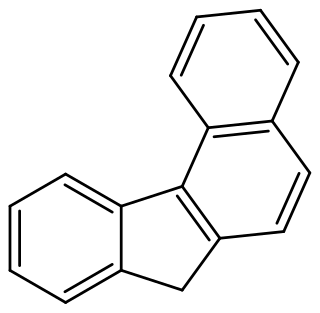
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.
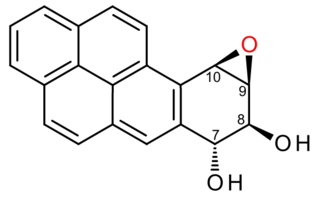
(+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is an organic compound with molecular formula C20H14O3. It is a metabolite and derivative of benzo[a]pyrene (found in tobacco smoke) as a result of oxidation to include hydroxyl and epoxide functionalities. (+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide binds to the N2 atom of a guanine nucleobase in DNA, distorting the double helix structure by intercalation of the pyrene moiety between base pairs through π-stacking. The carcinogenic properties of tobacco smoking are attributed in part to this compound binding and inactivating the tumor suppression ability of certain genes, leading to genetic mutations and potentially to cancer.




















