
Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

Sodium hypochlorite, commonly known in a dilute solution as (chlorine) bleach, is an alkaline inorganic chemical compound with the formula NaOCl, consisting of a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.

Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for human consumption, but water purification may also be carried out for a variety of other purposes, including medical, pharmacological, chemical, and industrial applications. The history of water purification includes a wide variety of methods. The methods used include physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination; and the use of electromagnetic radiation such as ultraviolet light.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach. More recent developments have extended its applications in food processing and as a disinfectant.

A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than sterilization, which is an extreme physical or chemical process that kills all types of life. Disinfectants are generally distinguished from other antimicrobial agents such as antibiotics, which destroy microorganisms within the body, and antiseptics, which destroy microorganisms on living tissue. Disinfectants are also different from biocides—the latter are intended to destroy all forms of life, not just microorganisms. Disinfectants work by destroying the cell wall of microbes or interfering with their metabolism. It is also a form of decontamination, and can be defined as the process whereby physical or chemical methods are used to reduce the amount of pathogenic microorganisms on a surface.

Hypochlorous acid is an acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine.

In chemistry, hypochlorite, or chloroxide is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite and calcium hypochlorite. The Cl-O distance in ClO− is 1.69 Å.
Shock chlorination is a process used in many swimming pools, water wells, springs, and other water sources to reduce the bacterial and algal residue in the water. Shock chlorination is performed by mixing a large amount of sodium hypochlorite, which can be in the form of a powder or a liquid such as chlorine bleach, into the water. The common advice is that the amount added must raise the level of chlorine to 10X the level of chloramines present in the pool water; this is "shocking". A lesser ratio is termed superchlorinating. Water that is being shock chlorinated should not be swum in or drunk until the sodium hypochlorite count in the water goes down to three ppm or less. Commercial sodium hypochlorite should not be mixed with commercial calcium hypochlorite, as there is a risk of explosion. Although a verb for superchlorination, "shock" is often misunderstood to be a unique type of product.
Calcium hypochlorite is an inorganic compound with formula Ca(ClO)2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent.
Salt water chlorination is a process that uses dissolved salt for the chlorination of swimming pools and hot tubs. The chlorine generator uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms, hypochlorous acid and sodium hypochlorite, which are already commonly used as sanitizing agents in pools. Hydrogen is produced as byproduct too.
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting point of −66 °C (−87 °F), but it is usually handled as a dilute aqueous solution, in which form it is sometimes used as a disinfectant. Chloramine is too unstable to have its boiling point measured.
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N−H bonds have been replaced by N−Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines. Chloramines are the most widely used members of the halamines.
Dakin's solution is a dilute solution of sodium hypochlorite and other stabilizing ingredients, traditionally used as an antiseptic, e.g. to cleanse wounds in order to prevent infection. The preparation was for a time called also Carrel–Dakin solution or Carrel–Dakin fluid.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove colour (whitening) from fabric or fiber or to disinfect after cleaning. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
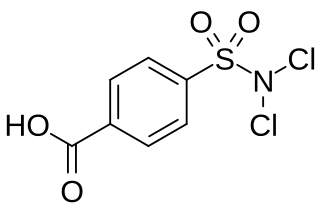
Halazone is a chemical compound whose formula can be written as either C
7H
5Cl
2NO
4S or (HOOC)(C
6H
4)(SO
2)(NCl
2). It has been widely used to disinfect drinking water.

Antoine Germain Labarraque was a French chemist and pharmacist, notable for formulating and finding important uses for "Eau de Labarraque" or "Labarraque's solution", a solution of sodium hypochlorite widely used as a disinfectant and deodoriser.

Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid.
Potassium hypochlorite is the potassium salt of hypochlorous acid. It is used in variable concentrations, often diluted in water solution. It has a light grey color and a strong chlorine smell. It can be used as a disinfectant.
A mixed oxidant solution (MOS) is a type of disinfectant that has many uses including disinfecting, sterilizing, and eliminating pathogenic microorganisms in water. An MOS may have advantages such as a higher disinfecting power, stable residual chlorine in water, elimination of biofilm, and safety. The main components of an MOS are chlorine and its derivatives, which are produced by electrolysis of sodium chloride. It may also contain high amounts of hydroxy radicals, chlorine dioxide, dissolved ozone, hydrogen peroxide and oxygen from which the name "mixed oxidant" is derived.

Liquid bleach, often called just bleach, is a common chemical household product that consists of a dilute solution of sodium hypochlorite and other secondary ingredients. It is a chlorine releasing bleaching agent widely used to whiten clothes and remove stains, as a disinfectant to kill germs, and for several other uses.











