
Hornblende is a complex inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common in igneous and metamorphic rocks.

Amphibole is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain SiO
4 tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is Amp. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.

The pyroxenes are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula XY(Si,Al)2O6, where X represents calcium (Ca), sodium (Na), iron or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron or. Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes.

Peridotite ( PERR-ih-doh-tyte, pə-RID-ə-) is a dense, coarse-grained igneous rock consisting mostly of the silicate minerals olivine and pyroxene. Peridotite is ultramafic, as the rock contains less than 45% silica. It is high in magnesium (Mg2+), reflecting the high proportions of magnesium-rich olivine, with appreciable iron. Peridotite is derived from Earth's mantle, either as solid blocks and fragments, or as crystals accumulated from magmas that formed in the mantle. The compositions of peridotites from these layered igneous complexes vary widely, reflecting the relative proportions of pyroxenes, chromite, plagioclase, and amphibole.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Jadeite is a pyroxene mineral with composition NaAlSi2O6. It is hard (Mohs hardness of about 6.5 to 7.0), very tough, and dense, with a specific gravity of about 3.4. It is found in a wide range of colors, but is most often found in shades of green or white. Jadeite is formed only in subduction zones on continental margins, where rock undergoes metamorphism at high pressure but relatively low temperature.

Enstatite is a mineral; the magnesium endmember of the pyroxene silicate mineral series enstatite (MgSiO3) – ferrosilite (FeSiO3). The magnesium rich members of the solid solution series are common rock-forming minerals found in igneous and metamorphic rocks. The intermediate composition, (Mg,Fe)SiO
3, has historically been known as hypersthene, although this name has been formally abandoned and replaced by orthopyroxene. When determined petrographically or chemically the composition is given as relative proportions of enstatite (En) and ferrosilite (Fs) (e.g., En80Fs20).

Ultramafic rocks are igneous and meta-igneous rocks with a very low silica content, generally >18% MgO, high FeO, low potassium, and are composed of usually greater than 90% mafic minerals. The Earth's mantle is composed of ultramafic rocks. Ultrabasic is a more inclusive term that includes igneous rocks with low silica content that may not be extremely enriched in Fe and Mg, such as carbonatites and ultrapotassic igneous rocks.

Komatiite is a type of ultramafic mantle-derived volcanic rock defined as having crystallised from a lava of at least 18 wt% MgO. Komatiites have low silicon, potassium and aluminium, and high to extremely high magnesium content. Komatiite was named for its type locality along the Komati River in South Africa, and frequently displays spinifex texture composed of large dendritic plates of olivine and pyroxene.

Melilite refers to a mineral of the melilite group. Minerals of the group are solid solutions of several endmembers, the most important of which are gehlenite and åkermanite. A generalized formula for common melilite is (Ca,Na)2(Al,Mg,Fe2+)[(Al,Si)SiO7]. Discovered in 1793 near Rome, it has a yellowish, greenish-brown color. The name derives from the Greek words meli (μέλι) "honey" and lithos (λίθους) "stone".The name refers to a group of minerals (melilite group) with chemically similar composition, nearly always minerals in åkermanite-gehlenite series.

Omphacite is a member of the clinopyroxene group of silicate minerals with formula: (Ca, Na)(Mg, Fe2+, Al)Si2O6. It is a variably deep to pale green or nearly colorless variety of clinopyroxene. It normally appears in eclogite, which is the high-pressure metamorphic rock of basalt. Omphacite is the solid solution of Fe-bearing diopside and jadeite. It crystallizes in the monoclinic system with prismatic, typically twinned forms, though usually anhedral. Its space group can be P2/n or C2/c depending on the thermal history. It exhibits the typical near 90° pyroxene cleavage. It is brittle with specific gravity of 3.29 to 3.39 and a Mohs hardness of 5 to 6.

Pigeonite is a mineral in the clinopyroxene subgroup of the pyroxene group. It has a general formula of (Ca,Mg,Fe)(Mg,Fe)Si2O6. The calcium cation fraction can vary from 5% to 25%, with iron and magnesium making up the rest of the cations.
Normative mineralogy is a calculation of the composition of a rock sample that estimates the idealised mineralogy of a rock based on a quantitative chemical analysis according to the principles of geochemistry.
Geothermobarometry is the science of measuring the previous pressure and temperature history of a metamorphic or intrusive igneous rocks. Geothermobarometry is a combination of geobarometry, where a pressure of mineral formation is resolved, and geothermometry where a temperature of formation is resolved.

The endmember hornblende tschermakite (☐Ca2(Mg3Al2)(Si6Al2)O22(OH)2) is a calcium rich monoclinic amphibole mineral. It is frequently synthesized along with its ternary solid solution series members tremolite and cummingtonite so that the thermodynamic properties of its assemblage can be applied to solving other solid solution series from a variety of amphibole minerals.
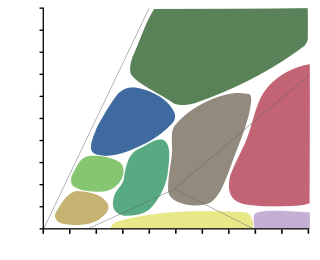
A metamorphic facies is a set of mineral assemblages in metamorphic rocks formed under similar pressures and temperatures. The assemblage is typical of what is formed in conditions corresponding to an area on the two dimensional graph of temperature vs. pressure. Rocks which contain certain minerals can therefore be linked to certain tectonic settings, times and places in the geological history of the area. The boundaries between facies are wide because they are gradational and approximate. The area on the graph corresponding to rock formation at the lowest values of temperature and pressure is the range of formation of sedimentary rocks, as opposed to metamorphic rocks, in a process called diagenesis.
Igneous petrology is the study of igneous rocks—those that are formed from magma. As a branch of geology, igneous petrology is closely related to volcanology, tectonophysics, and petrology in general. The modern study of igneous rocks utilizes a number of techniques, some of them developed in the fields of chemistry, physics, or other earth sciences. Petrography, crystallography, and isotopic studies are common methods used in igneous petrology.

Dorrite is a silicate mineral that is isostructural to the aenigmatite group. Although it is most chemically similar to the mineral rhönite [Ca2Mg5Ti(Al2Si4)O20], the lack of titanium (Ti) and presence of Fe3+ influenced dorrite's independence. Dorrite is named for Dr. John (Jack) A. Dorr, a late professor at the University of Michigan that researched in outcrops where dorrite was found in 1982. This mineral is sub-metallic resembling colors of brownish-black, dark brown, to reddish brown.

A petrogenetic grid is a geological phase diagram that connects the stability ranges or metastability ranges of metamorphic minerals or mineral assemblages to the conditions of metamorphism. Experimentally determined mineral or mineral-assemblage stability ranges are plotted as metamorphic reaction boundaries in a pressure–temperature cartesian coordinate system to produce a petrogenetic grid for a particular rock composition. The regions of overlap of the stability fields of minerals form equilibrium mineral assemblages used to determine the pressure–temperature conditions of metamorphism. This is particularly useful in geothermobarometry.
Garnet-Biotite Geothermometry is a method used to evaluate the peak temperature at which metamorphic rocks have formed. Geothermometry makes up one component of geothermobarometry, which also includes the evaluation of pressure (geobarometry). There are many geothermometers, but garnet-biotite is particularly useful because of the frequent occurrence of biotite and garnet together in medium grade metamorphic rocks. The garnet biotite thermometer correlates temperature with the partitioning of Fe and Mg in coinciding garnet and biotite. The garnet-biotite thermometer has been "calibrated" many times since the 70's by both experimental and empirical methods, however Ferry and Spear's 1978 experimental calibration study is reported thoroughly and commonly cited. Given a rock containing both garnet and biotite, an equilibrium constant (KD) can be found simply by using microprobe analysis. Then, by comparing the found KD value to the calculated garnet-biotite geothermometer, the peak temperature of rock formation can be determined.




















