
Cnidaria is a phylum under kingdom Animalia containing over 11,000 species of aquatic animals found both in fresh water and marine environments, including jellyfish, hydroids, sea anemones, corals and some of the smallest marine parasites. Their distinguishing features are a decentralized nervous system distributed throughout a gelatinous body and the presence of cnidocytes or cnidoblasts, specialized cells with ejectable flagella used mainly for envenomation and capturing prey. Their bodies consist of mesoglea, a non-living, jelly-like substance, sandwiched between two layers of epithelium that are mostly one cell thick. Cnidarians are also some of the only animals that can reproduce both sexually and asexually.

Sponges, the members of the phylum Porifera, are a basal animal clade as a sister of the diploblasts. They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like mesohyl sandwiched between two thin layers of cells.
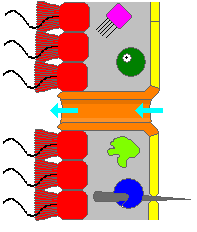
Choanocytes are cells that line the interior of asconoid, syconoid and leuconoid body types of sponges that contain a central flagellum, or cilium, surrounded by a collar of microvilli which are connected by a thin membrane.

Spongilla is a genus of freshwater sponges containing over 200 different species. Spongilla was first publicly recognized in 1696 by Leonard Plukenet and can be found in lakes, ponds and slow streams.Spongilla have a leuconoid body form with a skeleton composed of siliceous spicules. They are sessile organisms, attaching themselves to hard substrate like rocks, logs and sometimes to ground. Using their ostia and osculum these sponges filter the water for various small aquatic organisms such as protozoans, bacteria, and other free-floating pond life. Sponges of the genus Spongilla partake in symbiotic relationships with green algae, zoochlorellae. The symbiotic zoochlorellae give the sponges a green appearance and without them they would appear white.

Anheteromeyenia argyrosperma is a freshwater sponge found across North America.

Spawn is the eggs and sperm released or deposited into water by aquatic animals. As a verb, to spawn refers to the process of freely releasing eggs and sperm into a body of water ; the physical act is known as spawning. The vast majority of non-mammalian, non-avian and non-reptilian aquatic and/or amphibious lifeforms reproduce through this process, including the:
Arturia canariensis, commonly known as the yellow calcareous sponge, is a species of sponge in the family Clathrinidae. It is found in shallow seas in the Canary Islands, Cape Verde, the Adriatic Sea and the Caribbean Sea. The specific epithet "canariensis" was given to this species because it was first described from Lanzarote in the Canary Islands.
The choanoderm is a type of cell layer composed of flagellated collar cells, or choanocytes, found in sponges. The sponge body is mostly a connective tissue; the mesohyl, over which are applied epithelioid monolayers of cells, the outer pinacoderm and the inner choanoderm.

Callyspongia (Cladochalina) aculeata, commonly known as the branching vase sponge is a species of sea sponge in the family Callyspongiidae. Poriferans are typically characterized by ostia, pores that filter out plankton, with an osculum as the opening which water leaves through, and choanocytes trap food particles.
Mycale grandis, the orange keyhole sponge, is a species of marine demosponge in the family Mycalidae. Mycale is a large genus and this species is placed in the subgenus Mycale making its full name, Mycale (Mycale) grandis.

Crassadoma is a genus of rock scallops, marine bivalve molluscs in the family Pectinidae. It is monotypic, the only species being Crassadoma gigantea, the rock scallop, giant rock scallop or purple-hinge rock scallop. Although the small juveniles are free-swimming, they soon become sessile, and are cemented to the substrate. These scallops occur in the eastern Pacific Ocean.

Tectitethya crypta is a species of demosponge belonging to the family Tethyidae. Its classified family is characterized by fourteen different known genera, one of them being Tectitethya. It is a massive, shallow-water sponge found in the Caribbean Sea. This sponge was first discovered by Werner Bergmann in 1945 and later classified by de Laubenfels in 1949. It is located in reef areas situated on softer substrates such as sand or mud. Oftentimes, it is covered in sand and algae. This results in an appearance that is cream colored/ gray colored; however, when the animal is washed free of its sediment coverings, its body plan appears more green and gray. It's characterized with ostia peaking out of its body cavity, with the ability to abruptly open or close, changing its desired water flow rate through its mesohyl.
Cliona delitrix is a species of burrowing demosponge belonging to the family Clionaidae. It is found in shallow water in the Caribbean Sea and the Gulf of Mexico.
Agelas schmidti, commonly known as the brown tubular sponge, is a species of demosponge. It occurs at moderate depths in the Gulf of Mexico and the Caribbean Sea and often has a colonial coral growing over the surface. The type locality is Puerto Rico.
Neofibularia nolitangere, commonly known as the touch-me-not sponge, is a species of sea sponge in the family Biemnidae. It is found in shallow waters in the Western Atlantic Ocean and the Caribbean Sea.

Callyspongia truncata is a species of marine sea sponge. Like all marine sponges, C. truncata is a member of phylum Porifera and is defined by its filter-feeding lifestyle and flagellated choanocytes, or collar cells, that allow for water movement and feeding. It is a species of demosponge and a member of Demospongiae, the largest class of sponges as well as the family Callyspongiidae. C. truncata is most well known for being the organism from which the polyketide Callystatin A was identified. Callystatin A is a polyketide natural product from the leptomycin family of antibiotics. It was first isolated in 1997 from this organism, which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Recent studies have revealed numerous other bioactive compounds that have been found in this species.

Cliona viridis, commonly called the green boring sponge, is a species of demosponge in the family Clionaidae. Its form varies according to the nature of the surface on which it grows. In limestone and other calcareous substrates it excavates channels and chambers while on other types of rock it encrusts the surface or forms massive structures. It is native to the eastern Atlantic, the Mediterranean Sea and the Indo-Pacific Ocean.

Haliclona caerulea is a species of marine sponge in the family Chalinidae. It is an encrusting tubular sponge that grows anchored on rocky surfaces of coral reefs.
Dysidea etheria, commonly known as the ethereal sponge or heavenly sponge, is a species of lobate sponge within the class Demospongiae. This marine sponge is known for its light blue color and can be found in the Caribbean as well as off the coasts of Florida and Georgia. Like all other poriferans, D. etheria is capable of both sexual and asexual reproduction. The use of spicule collection as well as chemical defenses allows D. etheria to protect itself against predators such as the zebra doris and the orange knobby star. D. etheria is also known as a host species of the invasive brittle star Ophiothela mirabilis. Lastly, various molecular biology studies have utilized D. etheria to both study foreign particle transport in sponges and to isolate novel molecules.
Oscarella tuberculata is a species of sponge in the order Homosclerophorida. It is endemic to the Mediterranean Sea, where it forms encrusting colonies on rocks and other hard surfaces.
















