In chemistry, water of crystallization or water of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Cobalt(II) chloride is an inorganic compound of cobalt and chlorine, with the formula CoCl
2. It is a sky blue crystalline solid.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Naples yellow, also called antimony yellow, is an inorganic pigment used in paintings during the period 1700-1850. Colors range from a muted, or earthy, reddish yellow pigment to a bright light yellow. It is the chemical compound lead antimonate (Pb2Sb2O7). Also known as jaune d'antimoine, it is one of the oldest synthetic pigments. The Ancient Egyptians were known to create it.
Molybdenum trioxide is chemical compound with the formula MoO3. This compound is produced on the largest scale of any molybdenum compound. It is an intermediate in the production of molybdenum metal. It is also an important industrial catalyst. Molybdenum trioxide occurs as the rare mineral molybdite.

Tricalcium phosphate (sometimes abbreviated TCP) is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.

Cobalt(II) fluoride is a chemical compound with the formula (CoF2). It is a pink crystalline solid compound which is antiferromagnetic at low temperatures (TN=37.7 K) The formula is given for both the red tetragonal crystal, (CoF2), and the tetrahydrate red orthogonal crystal, (CoF2·4H2O). CoF2 is used in oxygen-sensitive fields, namely metal production. In low concentrations, it has public health uses. CoF2 is sparingly soluble in water. The compound can be dissolved in warm mineral acid, and will decompose in boiling water. Yet the hydrate is water-soluble, especially the di-hydrate CoF2·2H2 O and tri-hydrate CoF2·3H2O forms of the compound. The hydrate will also decompose with heat.
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.
Zirconium(IV) bromide is the inorganic compound with the formula ZrBr4. This colourless solid is the principal precursor to other Zr–Br compounds.

Cobalt(II) carbonate is the inorganic compound with the formula CoCO3. This reddish paramagnetic solid is an intermediate in the hydrometallurgical purification of cobalt from its ores. It is an inorganic pigment, and a precursor to catalysts. Cobalt(II) carbonate also occurs as the rare red/pink mineral spherocobaltite.

Sodium monothiophosphate, or sodium phosphorothioate, is a inorganic compound with the molecular formula Na3PO3S(H2O)x. All are white solids. The anhydrous material (x = 0) decomposes without melting at 120-125 °C. More common is the dodecahydrate. A nonahydrate is also known.
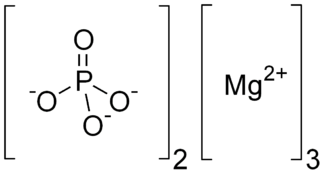
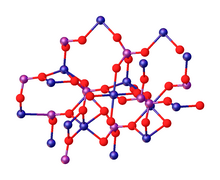
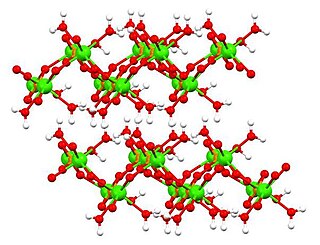
Trimagnesium phosphate describes inorganic compounds with formula Mg3(PO4)2.xH2O. They are magnesium acid salts of phosphoric acid, with varying amounts of water of crystallization: x = 0, 5, 8, 22.

Cobalt(II) bromide (CoBr2) is an inorganic compound. In its anhydrous form, it is a green solid that is soluble in water, used primarily as a catalyst in some processes.

Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula Co(OH)
2, consisting of divalent cobalt cations Co2+
and hydroxide anions HO−
. The pure compound, often called the "beta form" is a pink solid insoluble in water.

Niobium(V) fluoride, also known as niobium pentafluoride, is the inorganic compound]] with the formula NbF5. The solid consists of tetramers [NbF5]4. It is a colorless solid that is rarely used.

Chromium(II) sulfate refers to inorganic compounds with the chemical formula CrSO4·n H2O. Several closely related hydrated salts are known. The pentahydrate is a blue solid that dissolves readily in water. Solutions of chromium(II) are easily oxidized by air to Cr(III) species. Solutions of Cr(II) are used as specialized reducing agents of value in organic synthesis.

Chromium(III) phosphate describes inorganic compounds with the chemical formula CrPO4.(H2O)n, where n = 0, 4, or 6. All are deeply colored solids. Anhydrous CrPO4 is green. The hexahydrate CrPO4•6H2O is violet.
Monofluorophosphate is an anion with the formula PO3F2−, which is a phosphate group with one oxygen atom substituted with a fluoride atom. The charge of the ion is −2. The ion resembles sulfate in size, shape and charge, and can thus form compounds with the same structure as sulfates. These include Tutton's salts and langbeinites. The most well-known compound of monofluorophosphate is sodium monofluorophosphate, commonly used in toothpaste.

Titanyl sulfate is the inorganic compound with the formula TiOSO4. It is a white solid that forms by treatment of titanium dioxide with fuming sulfuric acid. It hydrolyzes to a gel of hydrated titanium dioxide. The structure consists of dense polymeric network with tetrahedral sulfur and octahedral titanium centers. The six ligands attached to titanium are derived from four different sulfate moieties and a bridging oxide. A monohydrate is also known, being prepared similarly to the anhydrous material. In the hydrate, one Ti–OS bond is replaced by Ti–OH2.

Aluminium dihydrogenphosphate describes inorganic compounds with the formula Al(H2PO4)3.xH2O where x = 0 or 3. They are white solids. Upon heating these materials convert sequentially to a family of related polyphosphate salts including aluminum triphosphate (AlH2P3O10.2H2O), aluminium hexametaphosphate (Al2P6O18), and aluminium tetrametaphosphate (Al4(P4O12)3). Some of these materials are used for fireproofing and as ingredients in specialized glasses.