An ascocarp, or ascoma, is the fruiting body (sporocarp) of an ascomycete phylum fungus. It consists of very tightly interwoven hyphae and millions of embedded asci, each of which typically contains four to eight ascospores. Ascocarps are most commonly bowl-shaped (apothecia) but may take on a spherical or flask-like form that has a pore opening to release spores (perithecia) or no opening (cleistothecia).

Chaetomium is a genus of fungi in the Chaetomiaceae family. It is a dematiaceous (dark-walled) mold normally found in soil, air, cellulose and plant debris. According to the Dictionary of the Fungi, there are about 95 species in the widespread genus.
Farrowia is a genus of fungi within the Chaetomiaceae family.

Chlamydosauromyces punctatus is the sole species in the monotypic genus of fungi, Chlamydosauromyces in the family, Onygenaceae. It was found in the skin shed from frilled lizard. This fungus is mesophilic and digests hair. It reproduces both sexually and asexually. The fungus has so far not been reported to be pathogenic.
Polytolypa is a monotypic genus of fungus containing the single species Polytolypa hystricis. First classified in the Onygenaceae family, as of 2008 it is considered to be in the Ajellomycetaceae, although there is still uncertainty as to its phylogenetic relationships with other similar genera. This species is only known from a single specimen derived in the laboratory from a specimen of dung of the North American porcupine, Erethizon dorsatum, collected in Ontario, Canada. Polytolypa hystricis contains bioactive compounds that have antifungal activity.
Chaetomium cupreum is a fungus in the family Chaetomiaceae. It is able to decay in manufactured cellulosic materials, and is known to antagonize a wide range of soil microorganisms. This species is component of the biocontrol agent, Ketomium, a commercial biofungicide. It has also been investigated for use in the production of natural dyes. Chaetomium cupreum is mesophilic and known to occur in harsh environments and can rapidly colonize organic substrates in soil. Laboratory cultures of C. cupreum can be propagated on a range of common growth media including potato dextrose at ambient or higher than ambient temperature producing cottony white colonies with a reddish reverse.
Massarina carolinensis is a species of fungus in the Lophiostomataceae family. The species is found exclusively on the lower parts of the culms of the saltmarsh Juncus roemerianus on the Atlantic Coast of North Carolina.
Paraphaeosphaeria pilleata is a species of fungus in the Lophiostomataceae family. The species fruits exclusively in the lower parts of the culms of the black needlerush. It is found on the Atlantic Coast of North Carolina.

Aphanoascus fulvescens is a mould fungus that behaves as a keratinophilic saprotroph and belongs to the Ascomycota. It is readily isolated from soil and dung containing keratin-rich tissues that have been separated from their animal hosts. This organism, distributed worldwide, is most commonly found in areas of temperate climate, in keeping with its optimal growth temperature of 28 °C (82 °F). While A. fulvescens is recognized as a geophilic fungal species, it is also a facultative opportunistic pathogen. Although it is not a dermatophyte, A. fulvescens has occasionally been shown to cause onychomycosis infections in humans. Its recognition in the laboratory is clinically important for correct diagnosis and treatment of human dermal infections.

Chaetomium globosum is a well-known mesophilic member of the mold family Chaetomiaceae. It is a saprophytic fungus that primarily resides on plants, soil, straw, and dung. Endophytic C. globosum assists in cellulose decomposition of plant cells. They are found in habitats ranging from forest plants to mountain soils across various biomes. C. globosum colonies can also be found indoors and on wooden products.
Thielavia subthermophila is a ubiquitous, filamentous fungus that is a member of the phylum Ascomycota and order Sordariales. Known to be found on plants of arid environments, it is an endophyte with thermophilic properties, and possesses dense, pigmented mycelium. Thielavia subthermophila has rarely been identified as a human pathogen, with a small number of clinical cases including ocular and brain infections. For treatment, antifungal drugs such as amphotericin B have been used topically or intravenously, depending upon the condition.
Chaetomium atrobrunneum is a darkly pigmented mould affiliated with the fungal division, Ascomycota. This species is predominantly saprotrophic, although it has been known to infect animals including humans, showing a proclivity for the tissues of the central nervous system. Chaetomium atrobrunneum was described in 1949 from a mouldy military mattress cover obtained from the island of Guadalcanal.
Botryotrichum murorum is a common soil and indoor fungus resembling members of the genus Chaetomium. The fungus has no known asexual state, and unlike many related fungi, is intolerant of high heat exhibiting limited growth when incubated at temperatures over 35 °C. In rare cases, the fungus is an opportunistic pathogen of marine animals and humans causing cutaneous and subcutaneous infection.
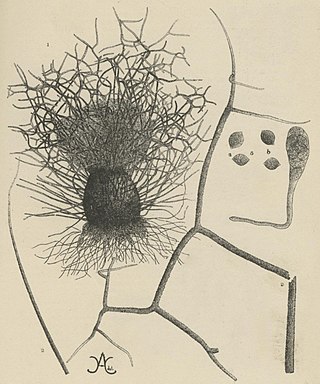
Chaetomium elatum is a very common and widely distributed saprotrophic fungus of the Chaetomiaceae family of molds which has been found to grow on many different substances all over the world. It was first established by Gustav Kunze after he observed it growing on dead leaves. Its defining features that distinguish it from other Chaetomium species are its extremely coarse terminal hairs and the lemon-shaped morphology of its ascospores. It produces many metabolites with potential biotechnology uses including one with promise against the rice blast disease fungus, Magnaporthe grisea. It shows very little pathogenic ability causing confirmed disease in only a few plant species.
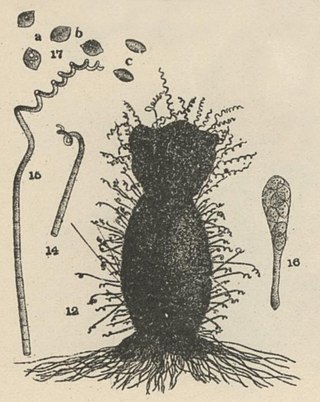
Chaetomium subspirale is a fungus from the phylum Ascomycota. It was described by A. H. Chivers in 1912 in America. The species has sexual fruiting bodies that are ornamented with characteristic, coiled hairs giving it a wooly appearance. C. subspirale colonies are brown, which the characteristic hairs are also responsible for. It is commonly found in various soil and dung samples. C. subspirale produces the mycotoxin, oxaspirodion, which inhibits inducible TNF-a expression and inhibits the activation of the transcription factor NF-kappaB.
Botryotrichum piluliferum is a fungal species first identified in 1885 by Saccardo and Marchal. It was discovered to be the asexual state of a member of the ascomycete genus, Chaetomium. The name B. piluliferum now applies to the fungus in all its states. B. piluliferum has been found worldwide in a wide range of habitats such as animal dung and vegetation. The colonies of this fungus start off white and grow rapidly to a brown colour. The conidia are smooth and white. B. piluliferum grows optimally at a temperature of 25-30 °C and a pH of 5.5.
Arcopilus aureus is a plant and soil fungus in the genus Arcopilus. It was first identified by A. H. Chivers in 1912, who named it Chaetomium aureum. It was later transferred to the genus Arcopilus by Wang and colleagues. The fungus has recently been recognized to have industrial use for the production of the metabolites resveratrol. and sclerotiorin Additionally, A. aureus has high lead tolerance and clearance, suggesting a potential role in environmental biotechnology.

Myxotrichum chartarum is a psychrophilic and cellulolytic fungus first discovered in Germany by Gustav Kunze in 1823. Its classification has changed many times over its history to better reflect the information available at the time. Currently, M. chartarum is known to be an ascomycete surrounded by a gymnothecium composed of ornate spines and releases asexual ascospores. The presence of cellulolytic processes are common in fungi within the family Myxotrichaceae. M. chartarum is one of many Myxotrichum species known to degrade paper and paper products. Evidence of M. chartarum "red spot" mold formation, especially on old books, can be found globally. As a result, this fungal species and other cellulolytic molds are endangering old works of art and books. Currently, there is no evidence that suggests that species within the family Myxotrichaceae are pathogenic.
Pseudothielavia terricola is a fungal species of the phylum Ascomycota, family Chaetomiaceae, and genus Pseudothielavia. Pseudothielavia terricola is widely distributed, especially in the tropical region of the world – with documented appearances in Africa, Southern Europe, and Asia. The species is mainly found in soil, but can also be found on other materials such as animal dung. The species was first assigned to the genus Coniothyrium in 1927, but was soon re-assigned to the genus Thielavia which endured for almost 90 years. Through intensive phylogenetic research and reassessment, the species was designated to a new genus, Pseudothielavia; the etymology of Pseudothielavia means similar to the genus Thielavia – the high resemblance was what contributed to the species assignment to the genus Thielavia nine decades ago. The fungus is mesophilic, grows abundantly in a pH level between 3.9–6, and is able to utilize multiple carbohydrates to support its growth. Mature Pseudothielavia terricola colonies in culture is dark brown in colour and spread out. Pseudothielavia terricola synthesizes a variety of compounds, two of which are thielavin A & B. These compounds were determined to be strong inhibitors of prostaglandin synthesis.
Cercophora areolata is a member of the Ascomycota division, and is grouped into the Lasiosphaeriaceae family based on morphology. C. areolata is a coprophilous fungus that has been most recently isolated from porcupine dung. Defining features of C. areolata include: 1) ovoid-conical, glabrous ascomata, 2) black, carbonaceous, areolate peridium and 3) clavate-shaped, single-walled asci. From studies on C. areolata, this fungus produces multiple antifungal compounds, which inhibit other competitor fungi.







