
Methylenetetrahydrofolate reductase (MTHFR) is the rate-limiting enzyme in the methyl cycle, and it is encoded by the MTHFR gene. Methylenetetrahydrofolate reductase catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation to methionine. Natural variation in this gene is common in otherwise healthy people. Although some variants have been reported to influence susceptibility to occlusive vascular disease, neural tube defects, Alzheimer's disease and other forms of dementia, colon cancer, and acute leukemia, findings from small early studies have not been reproduced. Some mutations in this gene are associated with methylenetetrahydrofolate reductase deficiency. Complex I deficiency with recessive spastic paraparesis has also been linked to MTHFR variants. In addition, the aberrant promoter hypermethylation of this gene is associated with male infertility and recurrent spontaneous abortion.
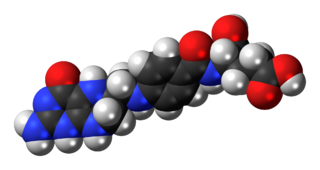
Tetrahydrofolic acid (THFA), or tetrahydrofolate, is a folic acid derivative.

Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme (EC 2.1.2.1) which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate (THF) to 5,10-methylenetetrahydrofolate (5,10-CH2-THF). This reaction provides the largest part of the one-carbon units available to the cell.

5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer [6R]-5,10-methylene-THF.

10-Formyltetrahydrofolate (10-CHO-THF) is a form of tetrahydrofolate that acts as a donor of formyl groups in anabolism. In these reactions 10-CHO-THF is used as a substrate in formyltransferase reactions.
In enzymology, sarcosine dehydrogenase (EC 1.5.8.3) is a mitochondrial enzyme that catalyzes the chemical reaction N-demethylation of sarcosine to give glycine. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-NH group of donor with other acceptors. The systematic name of this enzyme class is sarcosine:acceptor oxidoreductase (demethylating). Other names in common use include sarcosine N-demethylase, monomethylglycine dehydrogenase, and sarcosine:(acceptor) oxidoreductase (demethylating). Sarcosine dehydrogenase is closely related to dimethylglycine dehydrogenase, which catalyzes the demethylation reaction of dimethylglycine to sarcosine. Both sarcosine dehydrogenase and dimethylglycine dehydrogenase use FAD as a cofactor. Sarcosine dehydrogenase is linked by electron-transferring flavoprotein (ETF) to the respiratory redox chain. The general chemical reaction catalyzed by sarcosine dehydrogenase is:
In enzymology, a deoxycytidylate C-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a methylenetetrahydrofolate-tRNA-(uracil-5-)-methyltransferase (EC 2.1.1.74) is an enzyme that catalyzes the chemical reaction

In enzymology, a thymidylate synthase (FAD) (EC 2.1.1.148) is an enzyme that catalyzes the chemical reaction
In enzymology, a methylenetetrahydrofolate dehydrogenase (NAD+) (EC 1.5.1.15) is an enzyme that catalyzes a chemical reaction.

In enzymology, a methylenetetrahydrofolate dehydrogenase (NADP+) (EC 1.5.1.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a methylenetetrahydrofolate reductase (ferredoxin) (EC 1.5.7.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a formimidoyltetrahydrofolate cyclodeaminase (EC 4.3.1.4) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-methyl-2-oxobutanoate hydroxymethyltransferase (EC 2.1.2.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a D-alanine 2-hydroxymethyltransferase (EC 2.1.2.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a methionyl-tRNA formyltransferase (EC 2.1.2.9) is an enzyme that catalyzes the chemical reaction

In enzymology, a methenyltetrahydrofolate cyclohydrolase (EC 3.5.4.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a cytidylate kinase is an enzyme that catalyzes the chemical reaction

Methylenetetrahydrofolate dehydrogenase, cyclohydrolase and formyltetrahydrofolate synthetase 1 (MTHFD1) is a gene located in humans on chromosome 14 that encodes a protein, C-1-tetrahydrofolate synthase, cytoplasmic also known as C1-THF synthase, with three distinct enzymatic activities.
The glycine cleavage system (GCS) is also known as the glycine decarboxylase complex or GDC. The system is a series of enzymes that are triggered in response to high concentrations of the amino acid glycine. The same set of enzymes is sometimes referred to as glycine synthase when it runs in the reverse direction to form glycine. The glycine cleavage system is composed of four proteins: the T-protein, P-protein, L-protein, and H-protein. They do not form a stable complex, so it is more appropriate to call it a "system" instead of a "complex". The H-protein is responsible for interacting with the three other proteins and acts as a shuttle for some of the intermediate products in glycine decarboxylation. In both animals and plants, the glycine cleavage system is loosely attached to the inner membrane of the mitochondria. Mutations in this enzymatic system are linked with glycine encephalopathy.










