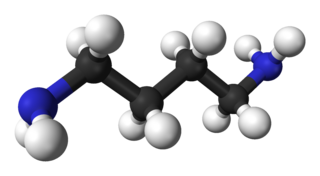
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors.
Spermine is a polyamine involved in cellular metabolism that is found in all eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. It functions as an intracellular free radical scavenger to protect DNA from free radical attack. Spermine is the chemical primarily responsible for the characteristic odor of semen.

Spermidine synthase is an enzyme that catalyzes the transfer of the propylamine group from S-adenosylmethioninamine to putrescine in the biosynthesis of spermidine. The systematic name is S-adenosyl 3-(methylthio)propylamine:putrescine 3-aminopropyltransferase and it belongs to the group of aminopropyl transferases. It does not need any cofactors. Most spermidine synthases exist in solution as dimers.
The enzyme adenosylmethionine decarboxylase catalyzes the conversion of S-adenosyl methionine to S-adenosylmethioninamine. Polyamines such as spermidine and spermine are essential for cellular growth under most conditions, being implicated in many cellular processes including DNA, RNA and protein synthesis. S-adenosylmethionine decarboxylase (AdoMetDC) plays an essential regulatory role in the polyamine biosynthetic pathway by generating the n-propylamine residue required for the synthesis of spermidine and spermine from putrescein. Unlike many amino acid decarboxylases AdoMetDC uses a covalently bound pyruvate residue as a cofactor rather than the more common pyridoxal 5'-phosphate. These proteins can be divided into two main groups which show little sequence similarity either to each other, or to other pyruvoyl-dependent amino acid decarboxylases: class I enzymes found in bacteria and archaea, and class II enzymes found in eukaryotes. In both groups the active enzyme is generated by the post-translational autocatalytic cleavage of a precursor protein. This cleavage generates the pyruvate precursor from an internal serine residue and results in the formation of two non-identical subunits termed alpha and beta which form the active enzyme.
In enzymology, a spermidine dehydrogenase (EC 1.5.99.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-ethylmalate synthase (EC 2.3.3.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a D-amino-acid N-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a D-tryptophan N-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a glucosamine-1-phosphate N-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a glutamate N-acetyltransferase (EC 2.3.1.35) is an enzyme that catalyzes the chemical reaction
In enzymology, a maltose O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a peptide alpha-N-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phenylalanine N-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a polysialic-acid O-acetyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a serine O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a homospermidine synthase (spermidine-specific) is an enzyme that catalyzes the chemical reaction

Diamine acetyltransferase 1 is an enzyme that in humans is encoded by the SAT1 gene found on the X chromosome.

Diamine acetyltransferase 2 is an enzyme that in humans is encoded by the SAT2 gene. SAT2 maintains a key metabolic glutamine/glutamate balance underpinning retrograde signaling by dendritic release of the neurotransmitter glutamate.
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature.
Non-specific polyamine oxidase (EC 1.5.3.17, polyamine oxidase, Fms1, AtPAO3) is an enzyme with systematic name polyamine:oxygen oxidoreductase (3-aminopropanal or 3-acetamidopropanal-forming). This enzyme catalyses the following chemical reaction