Related Research Articles

Magnesite is a mineral with the chemical formula MgCO
3. Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts.

Serpentinite is a metamorphic rock composed predominantly of one or more serpentine group minerals formed by near to complete serpentinization of mafic or ultramafic rocks. Its name originated from the similarity of the texture of the rock to that of the skin of a snake. Serpentinite has been called serpentine or serpentine rock, particularly in older geological texts and in wider cultural settings.

Serpentinization is a hydration and metamorphic transformation of ferromagnesian minerals, such as olivine and pyroxene, in mafic and ultramafic rock to produce serpentinite. Minerals formed by serpentinization include the serpentine group minerals, brucite, talc, Ni-Fe alloys, and magnetite. The mineral alteration is particularly important at the sea floor at tectonic plate boundaries.

The Lost City Hydrothermal Field, often referred to simply as Lost City, is an area of marine alkaline hydrothermal vents located on the Atlantis Massif at the intersection between the Mid-Atlantic Ridge and the Atlantis Transform Fault, in the Atlantic Ocean. It is a long-lived site of active and inactive ultramafic-hosted serpentinization, abiotically producing many simple molecules such as methane and hydrogen which are fundamental to microbial life. As such it has generated scientific interest as a prime location for investigating the origin of life on Earth and other planets similar to it.

The hydrogen cycle consists of hydrogen exchanges between biotic (living) and abiotic (non-living) sources and sinks of hydrogen-containing compounds.
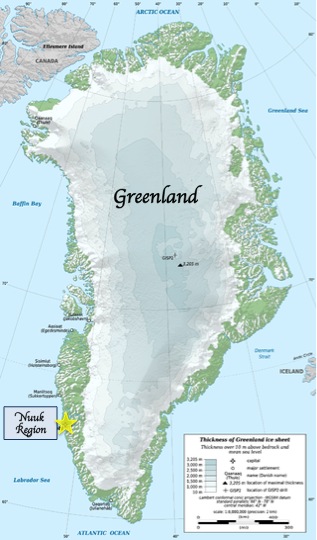
The Isua Greenstone Belt is an Archean greenstone belt in southwestern Greenland, aged between 3.7 and 3.8 billion years. The belt contains variably metamorphosed mafic volcanic and sedimentary rocks, and is the largest exposure of Eoarchaean supracrustal rocks on Earth. Due to its age and low metamorphic grade relative to many Eoarchaean rocks, the Isua Greenstone Belt has become a focus for investigations on the emergence of life and the style of tectonics that operated on the early Earth.
The Guaymas Basin is the largest marginal rift basin located in the Gulf of California. It made up of the northern and southern trough and is linked to the Guaymas Fault to the north and the Carmen Fault to the south. The mid-ocean ridge system is responsible for the creation of the Guaymas Basin and giving it many features such as hydrothermal circulation and hydrocarbon seeps. Hydrothermal circulation is a significant process in the Guaymas Basin because it recycles energy and nutrients which are instrumental in sustaining the basin's rich ecosystem. Additionally, hydrocarbons and other organic matter are needed to feed a variety of organisms, many of which have adapted to tolerate the basin's high temperatures.

The class Zetaproteobacteria is the sixth and most recently described class of the Pseudomonadota. Zetaproteobacteria can also refer to the group of organisms assigned to this class. The Zetaproteobacteria were originally represented by a single described species, Mariprofundus ferrooxydans, which is an iron-oxidizing neutrophilic chemolithoautotroph originally isolated from Kamaʻehuakanaloa Seamount in 1996 (post-eruption). Molecular cloning techniques focusing on the small subunit ribosomal RNA gene have also been used to identify a more diverse majority of the Zetaproteobacteria that have as yet been unculturable.
Geochemical modeling or theoretical geochemistry is the practice of using chemical thermodynamics, chemical kinetics, or both, to analyze the chemical reactions that affect geologic systems, commonly with the aid of a computer. It is used in high-temperature geochemistry to simulate reactions occurring deep in the Earth's interior, in magma, for instance, or to model low-temperature reactions in aqueous solutions near the Earth's surface, the subject of this article.

Frederick T. Mackenzie was an American sedimentary and global biogeochemist. Mackenzie applied experimental and field data coupled to a sound theoretical framework to the solution of geological, geochemical, and oceanographic problems at various time and space scales.
Clumped isotopes are heavy isotopes that are bonded to other heavy isotopes. The relative abundance of clumped isotopes (and multiply-substituted isotopologues) in molecules such as methane, nitrous oxide, and carbonate is an area of active investigation. The carbonate clumped-isotope thermometer, or "13C–18O order/disorder carbonate thermometer", is a new approach for paleoclimate reconstruction, based on the temperature dependence of the clumping of 13C and 18O into bonds within the carbonate mineral lattice. This approach has the advantage that the 18O ratio in water is not necessary (different from the δ18O approach), but for precise paleotemperature estimation, it also needs very large and uncontaminated samples, long analytical runs, and extensive replication. Commonly used sample sources for paleoclimatological work include corals, otoliths, gastropods, tufa, bivalves, and foraminifera. Results are usually expressed as Δ47 (said as "cap 47"), which is the deviation of the ratio of isotopologues of CO2 with a molecular weight of 47 to those with a weight of 44 from the ratio expected if they were randomly distributed.

Dimitri Alexander Sverjensky is a professor in Earth and Planetary Sciences at Johns Hopkins University where his research is focused on geochemistry.
Shuhei Ono is a professor of earth, atmospheric, and planetary sciences at the Massachusetts Institute of Technology. In his research, he measures isotopes of sulfur and other elements to investigate water-rock-microbe interactions, seafloor hydrothermal systems, the deep biosphere, and global sulfur cycles.
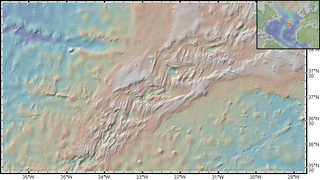
The Rainbow hydrothermal vent field is a system of ultramafic-hosted hydrothermal vents located at 36°14'N on the Mid-Atlantic Ridge (MAR). It was discovered in 1994 from temperature readings of ten high-temperature black smokers at a depth of approximately 2.3 kilometres (1.4 mi), where fluids can exceed 365 °C (689 °F). The site is shallower and larger in area than many other vent fields along the Azores section of the MAR with an area of 1.5 square kilometres. Located 370 km (229.91 mi) southeast of Faial Island, it is a popular geochemical sampling and modeling site due to close proximity to the Azores and definitive representation of serpentinization from hydrothermal circulation and synthesis.
Carbonate-associated sulfates (CAS) are sulfate species found in association with carbonate minerals, either as inclusions, adsorbed phases, or in distorted sites within the carbonate mineral lattice. It is derived primarily from dissolved sulfate in the solution from which the carbonate precipitates. In the ocean, the source of this sulfate is a combination of riverine and atmospheric inputs, as well as the products of marine hydrothermal reactions and biomass remineralisation. CAS is a common component of most carbonate rocks, having concentrations in the parts per thousand within biogenic carbonates and parts per million within abiogenic carbonates. Through its abundance and sulfur isotope composition, it provides a valuable record of the global sulfur cycle across time and space.
In stable isotope geochemistry, the Urey–Bigeleisen–Mayer equation, also known as the Bigeleisen–Mayer equation or the Urey model, is a model describing the approximate equilibrium isotope fractionation in an isotope exchange reaction. While the equation itself can be written in numerous forms, it is generally presented as a ratio of partition functions of the isotopic molecules involved in a given reaction. The Urey–Bigeleisen–Mayer equation is widely applied in the fields of quantum chemistry and geochemistry and is often modified or paired with other quantum chemical modelling methods to improve accuracy and precision and reduce the computational cost of calculations.
Susan Humphris is a geologist known for her research on processes at mid-ocean ridges. She is an elected fellow of the American Geophysical Union.
Minoru Ozima is a geochemist and Professor Emeritus of the Department of Earth and Planetary Science, Graduate School of Science, at the University of Tokyo. He was named one of the top 100 Asian scientists for the year 2021 by Asian Scientist magazine.
Hilairy Ellen Hartnett is professor at Arizona State University known for her work on biogeochemical processes in modern and paleo-environments.
Hugh Pettingill Taylor Jr. was an American geochemist.
References
- ↑ "Everett Shock". American Geophysical Union. Retrieved 17 December 2022.
- ↑ "Geochemistry Fellows". Geochemical Society. Retrieved 17 December 2022.
- 1 2 3 4 5 6 "Everett Shock". Arizona State University. Retrieved 29 September 2022.
- 1 2 3 4 5 6 You Don't Know Mojack (19 September 2021). "182 Everett Shock "Ghost Boys" w/ Everett Shock" (Podcast). Retrieved 28 September 2022.
- ↑ Shock, Everett L.; Helgeson, Harold C. (1988). "Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000°C". Geochimica et Cosmochimica Acta. 52 (8): 2009–2036. Bibcode:1988GeCoA..52.2009S. doi:10.1016/0016-7037(88)90181-0.
- ↑ Shock, Everett L.; Helgeson, Harold C. (1990). "Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Standard partial molal properties of organic species". Geochimica et Cosmochimica Acta. 54 (4): 915–945. Bibcode:1990GeCoA..54..915S. doi:10.1016/0016-7037(90)90429-O.
- 1 2 3 "Everett Shock". NASA. 27 February 2019. Retrieved 29 September 2022.
- ↑ Cassis, Nikki (15 October 2008). "Research points to methods for recovering petroleum". ASU News. Retrieved 18 December 2022.
- ↑ Klemaszewski, James (6 July 2022). "ASU researchers patent a new industrial-scale chemical method using geomimicry". ASU News. Retrieved 18 December 2022.
- ↑ Shock, Everett L.; Holland, Melanie; Meyer-Dombard, D'arcy; Amend, Jan P.; Osburn, G.R.; Fischer, Tobias P. (2010). "Quantifying inorganic sources of geochemical energy in hydrothermal ecosystems, Yellowstone National Park, USA". Geochimica et Cosmochimica Acta. 74 (14): 4005–4043. Bibcode:2010GeCoA..74.4005S. doi:10.1016/j.gca.2009.08.036.
- ↑ Reichard, Sean (3 July 2017). "Researchers Puzzle Over Unconventional Yellowstone Microbe". Yellowstone Insider. Retrieved 18 December 2022.
- ↑ Amenabar, Maximiliano J.; Shock, Everett L.; Roden, Eric E.; Peters, John W.; Boyd, Eric S. (2017). "Microbial substrate preference dictated by energy demand rather than supply". Nature Geoscience. 10: 577–581. doi:10.1038/NGEO2798.
- ↑ Boyer, Grayson M.; Schubotz, Florence; Summons, Roger E.; Woods, Jade; Shock, Everett L. (2020). "Carbon Oxidation State in Microbial Polar Lipids Suggests Adaptation to Hot Spring Temperature and Redox Gradients". Frontiers in Microbiology. 11: 229. Bibcode:2020FrMic..11..229B. doi: 10.3389/fmicb.2020.00229 . PMC 7044123 . PMID 32153529.
- ↑ Sidder, Aaron (25 February 2022). "A Fresh View of Microbial Life in Yellowstone's Hot Springs". Eos. American Geophysical Union. Retrieved 16 December 2022.
- ↑ Fecteau, Kristopher M.; Boyd, Eric S.; Lindsay, Melody R.; Amenabar, Maximiliano J.; Robinson, Kirtland J.; Debes II, R. Vincent; Shock, Everett L. (2022). "Cyanobacteria and Algae Meet at the Limits of Their Habitat Ranges in Moderately Acidic Hot Springs". Journal of Geophysical Research: Biogeosciences. 127 (1). Bibcode:2022JGRG..12706446F. doi:10.1029/2021JG006446. S2CID 240874714.
- ↑ Osburn, Magadlena R.; Amend, Jan P. (2011). "Thermogladius shockii gen. nov., sp. nov., a hyperthermophilic crenarchaeote from Yellowstone National Park, USA". Archives of Microbiology. 193 (1): 45–52. Bibcode:2011ArMic.193...45O. doi:10.1007/s00203-010-0639-8. PMID 20978744. S2CID 2762893.
- ↑ Green, Jenny (20 December 2010). "New species of archaeon named after ASU professor". ASU News. Retrieved 30 September 2022.
- ↑ Valentine, Karin (25 April 2022). "Scientists study microorganisms on Earth to gain insight into life on other planets". ASU News. Retrieved 16 December 2022.
- ↑ Howells, Alta E. G.; Leong, James A. M.; Ely, Tucker; Santana, Michelle; Robinson, Kirt; Esquivel-Elizondo, Sofia; Cox, Alysia; Poret-Peterson, Amisha; Krajmaknik-Brown, Rosa; Shock, Everett L. (2022). "Energetically Informed Niche Models of Hydrogenotrophs Detected in Sediments of Serpentinized Fluids of the Samail Ophiolite of Oman". Journal of Geophysical Research: Biogeosciences. 127 (3). Bibcode:2022JGRG..12706317H. doi:10.1029/2021JG006317. S2CID 247161798.
- ↑ Valentine, Karin (14 January 2022). "Weathering rocks hold clues to Earth's Great Oxidation Event". ASU News. Retrieved 16 December 2022.
- ↑ Leong, James Andrew M.; Ely, Tucker; Shock, Everett L. (2021). "Decreasing extents of Archean serpentinization contributed to the rise of an oxidized atmosphere". Nature Communications. 12 (1): 7341. Bibcode:2021NatCo..12.7341L. doi:10.1038/s41467-021-27589-7. PMC 8688491 . PMID 34930924.
- ↑ Mack, Eric. "Extreme deep-sea conditions could support life on Earth, other alien worlds". CNET. Retrieved 1 October 2022.
- ↑ Derouin, Sarah (7 January 2022). "Hydrothermal Microbes Can Be Green Energy Producers". Eos. American Geophysical Union. Retrieved 16 December 2022.
- ↑ Dick, Jeffrey; Shock, Everett L. (2021). "The Release of Energy During Protein Synthesis at Ultramafic-Hosted Submarine Hydrothermal Ecosystems". Journal of Geophysical Research: Biogeosciences. 126 (11). Bibcode:2021JGRG..12606436D. doi:10.1029/2021JG006436. S2CID 240324503.
- ↑ Ionescu, Andrei. "Deep sea vents are favorable to the formation of life". earth.com. Retrieved 6 October 2022.
- 1 2 Latella, Chris (31 March 2021). "ASU researchers using deep-sea exploration to learn about space". 12news. Retrieved 6 October 2022.
- ↑ Seckel, Scott (13 June 2019). "Boldly going where no one has gone before — on Earth". ASU News. Retrieved 6 October 2022.
- ↑ Mcclay, Bob (31 May 2015). "ASU scientists developing instruments for NASA mission". KTAR News. Retrieved 6 October 2022.