
Color blindness is the decreased ability to see color or differences in color. It can impair tasks such as selecting ripe fruit, choosing clothing, and reading traffic lights. Color blindness may make some academic activities more difficult. However, issues are generally minor, and the colorblind automatically develop adaptations and coping mechanisms. People with total color blindness (achromatopsia) may also be uncomfortable in bright environments and have decreased visual acuity.

Color vision, a feature of visual perception, is an ability to perceive differences between light composed of different wavelengths independently of light intensity. Color perception is a part of the larger visual system and is mediated by a complex process between neurons that begins with differential stimulation of different types of photoreceptors by light entering the eye. Those photoreceptors then emit outputs that are propagated through many layers of neurons and then ultimately to the brain. Color vision is found in many animals and is mediated by similar underlying mechanisms with common types of biological molecules and a complex history of evolution in different animal taxa. In primates, color vision may have evolved under selective pressure for a variety of visual tasks including the foraging for nutritious young leaves, ripe fruit, and flowers, as well as detecting predator camouflage and emotional states in other primates.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.

Tetrachromacy is the condition of possessing four independent channels for conveying color information, or possessing four types of cone cell in the eye. Organisms with tetrachromacy are called tetrachromats.

Trichromacy or trichromatism is the possessing of three independent channels for conveying color information, derived from the three different types of cone cells in the eye. Organisms with trichromacy are called trichromats.
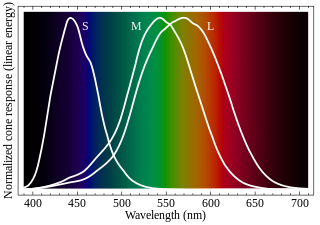
Cone cells, or cones, are photoreceptor cells in the retinas of vertebrate eyes including the human eye. They respond differently to light of different wavelengths, and the combination of their responses is responsible for color vision. Cones function best in relatively bright light, called the photopic region, as opposed to rod cells, which work better in dim light, or the scotopic region. Cone cells are densely packed in the fovea centralis, a 0.3 mm diameter rod-free area with very thin, densely packed cones which quickly reduce in number towards the periphery of the retina. Conversely, they are absent from the optic disc, contributing to the blind spot. There are about six to seven million cones in a human eye, with the highest concentration being towards the macula.
In visual physiology, adaptation is the ability of the retina of the eye to adjust to various levels of light. Natural night vision, or scotopic vision, is the ability to see under low-light conditions. In humans, rod cells are exclusively responsible for night vision as cone cells are only able to function at higher illumination levels. Night vision is of lower quality than day vision because it is limited in resolution and colors cannot be discerned; only shades of gray are seen. In order for humans to transition from day to night vision they must undergo a dark adaptation period of up to two hours in which each eye adjusts from a high to a low luminescence "setting", increasing sensitivity hugely, by many orders of magnitude. This adaptation period is different between rod and cone cells and results from the regeneration of photopigments to increase retinal sensitivity. Light adaptation, in contrast, works very quickly, within seconds.

Monochromacy is the ability of organisms or machines to perceive only light intensity, without respect to spectral composition (color). Organisms with monochromacy are called monochromats.

Photopsins are the photoreceptor proteins found in the cone cells of the retina that are the basis of color vision. Photopsins bind the chromophore retinal to form iodopsins. Iodopsins are used in daylight vision and are analogous to rhodopsin that is used in night vision.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.

Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via the chromophore retinal. Most prominently, they are found in photoreceptor cells of the retina. Five classical groups of opsins are involved in vision, mediating the conversion of a photon of light into an electrochemical signal, the first step in the visual transduction cascade. Another opsin found in the mammalian retina, melanopsin, is involved in circadian rhythms and pupillary reflex but not in vision. Humans have in total nine opsins. Beside vision and light perception, opsins may also sense temperature, sound, and chemicals.
Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are rhodopsin in the photoreceptor cells of the vertebrate retina, phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. They mediate light responses as varied as visual perception, phototropism and phototaxis, as well as responses to light-dark cycles such as circadian rhythm and other photoperiodisms including control of flowering times in plants and mating seasons in animals.

Blue-sensitive opsin is a protein that in humans is encoded by the OPN1SW gene.

Opsin-5, also known as G-protein coupled receptor 136 or neuropsin is a protein that in humans is encoded by the OPN5 gene. Opsin-5 is a member of the opsin subfamily of the G protein-coupled receptors. It is a photoreceptor protein sensitive to ultraviolet (UV) light. The OPN5 gene was discovered in mouse and human genomes and its mRNA expression was also found in neural tissues. Neuropsin is bistable at 0 °C and activates a UV-sensitive, heterotrimeric G protein Gi-mediated pathway in mammalian and avian tissues.

Green-sensitive opsin is a protein that in humans is encoded by the OPN1MW gene. OPN1MW2 is a similar opsin.

OPN1LW is a gene on the X chromosome that encodes for long wave sensitive (LWS) opsin, or red cone photopigment. It is responsible for perception of visible light in the yellow-green range on the visible spectrum. The gene contains 6 exons with variability that induces shifts in the spectral range. OPN1LW is subject to homologous recombination with OPN1MW, as the two have very similar sequences. These recombinations can lead to various vision problems, such as red-green colourblindness and blue monochromacy. The protein encoded is a G-protein coupled receptor with embedded 11-cis-retinal, whose light excitation causes a cis-trans conformational change that begins the process of chemical signalling to the brain.

The evolution of color vision in primates is highly unusual compared to most eutherian mammals. A remote vertebrate ancestor of primates possessed tetrachromacy, but nocturnal, warm-blooded, mammalian ancestors lost two of four cones in the retina at the time of dinosaurs. Most teleost fish, reptiles and birds are therefore tetrachromatic while most mammals are strictly dichromats, the exceptions being some primates and marsupials, who are trichromats, and many marine mammals, who are monochromats.
Color vision, a proximate adaptation of the vision sensory modality, allows for the discrimination of light based on its wavelength components.

Vision is the most important sense for birds, since good eyesight is essential for safe flight. Birds have a number of adaptations which give visual acuity superior to that of other vertebrate groups; a pigeon has been described as "two eyes with wings". Birds are theropod dinosaurs, and the avian eye resembles that of other reptiles, with ciliary muscles that can change the shape of the lens rapidly and to a greater extent than in the mammals. Birds have the largest eyes relative to their size in the animal kingdom, and movement is consequently limited within the eye's bony socket. In addition to the two eyelids usually found in vertebrates, bird's eyes are protected by a third transparent movable membrane. The eye's internal anatomy is similar to that of other vertebrates, but has a structure, the pecten oculi, unique to birds.
Blue cone monochromacy (BCM) is an inherited eye disease that causes severely impaired color discrimination, low vision, nystagmus and photophobia due to the absence of functionality of red (L) and green (M) cone photoreceptor cells in the retina. This form of retinal disorder is a recessive X-linked disease and manifests its symptoms in early infancy.














