
Beta-lactamases (β-lactamases) are enzymes produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.

Gentamicin is an antibiotic used to treat several types of bacterial infections. This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others. It is not effective for gonorrhea or chlamydia infections. It can be given intravenously, by intramuscular injection, or topically. Topical formulations may be used in burns or for infections of the outside of the eye. It is often only used for two days until bacterial cultures determine what specific antibiotics the infection is sensitive to. The dose required should be monitored by blood testing.
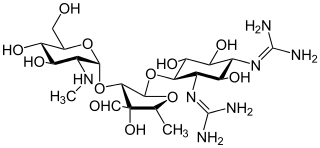
Aminoglycoside is a medicinal and bacteriologic category of traditional Gram-negative antibacterial medications that inhibit protein synthesis and contain as a portion of the molecule an amino-modified glycoside (sugar). The term can also refer more generally to any organic molecule that contains amino sugar substructures. Aminoglycoside antibiotics display bactericidal activity against Gram-negative aerobes and some anaerobic bacilli where resistance has not yet arisen but generally not against Gram-positive and anaerobic Gram-negative bacteria.

Piperacillin is a broad-spectrum β-lactam antibiotic of the ureidopenicillin class. The chemical structure of piperacillin and other ureidopenicillins incorporates a polar side chain that enhances penetration into Gram-negative bacteria and reduces susceptibility to cleavage by Gram-negative beta lactamase enzymes. These properties confer activity against the important hospital pathogen Pseudomonas aeruginosa. Thus piperacillin is sometimes referred to as an "anti-pseudomonal penicillin".

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes.

Tobramycin is an aminoglycoside antibiotic derived from Streptomyces tenebrarius that is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas.

Amikacin is an antibiotic medication used for a number of bacterial infections. This includes joint infections, intra-abdominal infections, meningitis, pneumonia, sepsis, and urinary tract infections. It is also used for the treatment of multidrug-resistant tuberculosis. It is used by injection into a vein using an IV or into a muscle.
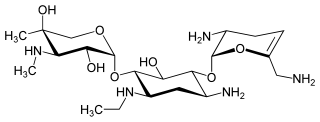
Netilmicin (1-N-ethylsisomicin) is a semisynthetic aminoglycoside antibiotic, and a derivative of sisomicin, produced by Micromonospora inyoensis. Aminoglycoside antibiotics have the ability to kill a wide variety of bacteria. Netilmicin is not absorbed from the gut and is therefore only given by injection or infusion. It is only used in the treatment of serious infections particularly those resistant to gentamicin.

In enzymology, an acetyl-CoA C-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an aminoglycoside N3'-acetyltransferase (EC 2.3.1.81) is an enzyme that catalyzes the chemical reaction
In enzymology, an aminoglycoside N6'-acetyltransferase (EC 2.3.1.82) is an enzyme that catalyzes the chemical reaction
In enzymology, a gentamicin 2'-N-acetyltransferase (EC 2.3.1.59) is an enzyme that catalyzes the chemical reaction

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a peptide alpha-N-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a gentamicin 2"-nucleotidyltransferase is an enzyme that catalyzes the chemical reaction
Aminoglycoside-3'-phosphotransferase, also known as aminoglycoside kinase, is an enzyme that primarily catalyzes the addition of phosphate from ATP to the 3'-hydroxyl group of a 4,6-disubstituted aminoglycoside, such as kanamycin. However, APH(3') has also been found to phosphorylate at the 5'-hydroxyl group in 4,5-disubstituted aminoglycosides, which lack a 3'-hydroxyl group, and to diphosphorylate hydroxyl groups in aminoglycosides that have both 3'- and 5'-hydroxyl groups. Primarily positively charged at biological conditions, aminoglycosides bind to the negatively charged backbone of nucleic acids to disrupt protein synthesis, effectively inhibiting bacterial cell growth. APH(3') mediated phosphorylation of aminoglycosides effectively disrupts their mechanism of action, introducing a phosphate group that reduces their binding affinity due to steric hindrances and unfavorable electrostatic interactions. APH(3') is primarily found in certain species of gram-positive bacteria.

Sisomicin, is an aminoglycoside antibiotic, isolated from the fermentation broth of Micromonospora inositola. It is a newer broad-spectrum aminoglycoside most structurally related to gentamicin.
UDP-2-acetamido-3-amino-2,3-dideoxy-glucuronate N-acetyltransferase is an enzyme with systematic name acetyl-CoA:UDP-2-acetamido-3-amino-2,3-dideoxy-alpha-D-glucuronate N-acetyltransferase. This enzyme catalyses the following chemical reaction

Plazomicin, sold under the brand name Zemdri, is an aminoglycoside antibiotic used to treat complicated urinary tract infections. As of 2019 it is recommended only for those in whom alternatives are not an option. It is given by injection into a vein.

Nourseothricin (NTC) is a member of the streptothricin-class of aminoglycoside antibiotics produced by Streptomyces species. Chemically, NTC is a mixture of the related compounds streptothricin C, D, E, and F. NTC inhibits protein synthesis by inducing miscoding. It is used as a selection marker for a wide range of organisms including bacteria, yeast, filamentous fungi, and plant cells. It is not known to have adverse side-effects on positively selected cells, a property cardinal to a selection drug.














