
A steroid is an organic compound with four fused rings arranged in a specific molecular configuration.
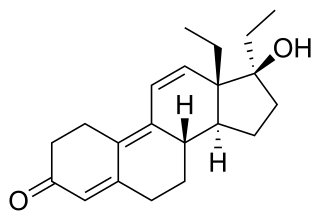
Tetrahydrogestrinone (THG), known by the nickname The Clear, is a synthetic and orally active anabolic–androgenic steroid (AAS) which was never marketed for medical use. It was developed by Patrick Arnold and was used by a number of high-profile athletes such as Barry Bonds and Dwain Chambers.

Quinbolone, sold under the brand names Anabolicum and Anabolvis, is an androgen and anabolic steroid (AAS) which was previously marketed in Italy. It was developed by Parke-Davis as a viable orally administered AAS with little or no liver toxicity.

Luis Ernesto Miramontes Cárdenas was a Mexican chemist known as co-inventor and the first to synthesize an oral contraceptive, progestin norethisterone.

Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane and 5β-pregnane (17β-ethyletiocholane). It has a gonane core.

Metribolone is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was never marketed for medical use but has been widely used in scientific research as a hot ligand in androgen receptor (AR) ligand binding assays (LBAs) and as a photoaffinity label for the AR. More precisely, metribolone is the 17α-methylated derivative of trenbolone. It was investigated briefly for the treatment of advanced breast cancer in women in the late 1960s and early 1970s, but was found to produce signs of severe hepatotoxicity at very low dosages, and its development was subsequently discontinued.

Azacosterol, or azacosterol hydrochloride, also known as 20,25-diazacholesterol, is a cholesterol-lowering drug (hypocholesteremic), which was marketed previously, but has since been discontinued. It is also an avian chemosterilant used to control pest pigeon populations via inducing sterility. The drug is a sterol and derivative of cholesterol in which two carbon atoms have been replaced with nitrogen atoms.

Methandriol, also known as methylandrostenediol, is an androgen and anabolic steroid (AAS) medication which was developed by Organon and is used in both oral and injectable formulations. It is an orally active 17α-alkylated AAS and a derivative of the endogenous androgen prohormone androstenediol.

Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed. It is taken by mouth.

Trimegestone, sold under the brand names Ondeva and Totelle among others, is a progestin medication which is used in menopausal hormone therapy and in the prevention of postmenopausal osteoporosis. It was also under development for use in birth control pills to prevent pregnancy, but ultimately was not marketed for this purpose. The medication is available alone or in combination with an estrogen. It is taken by mouth.

Moxestrol, sold under the brand name Surestryl, is an estrogen medication which has been used in Europe for the treatment of menopausal symptoms and menstrual disorders. It is taken by mouth. In addition to its use as a medication, moxestrol has been used in scientific research as a radioligand of the estrogen receptor.

Estrane is a C18 steroid derivative, with a gonane core.

Rosterolone, also known as 17α-propylmesterolone or 1α-methyl-17α-propyl-5α-androstan-17β-ol-3-one, is a steroidal antiandrogen which was first described in 1984 and was developed for topical administration but was never marketed. It has shown some efficacy in the treatment of acne, and lacks systemic effects with either topical or systemic administration. Rosterolone is a derivative of mesterolone, which, in contrast, is an androgen and anabolic steroid.

Orestrate, also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed. It is the C3 propionate ester and C17β-(1-cyclohexenyl) ether of estradiol.

Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.

Acetomepregenol (ACM), also known as mepregenol diacetate and sold under the brand name Diamol, is a progestin medication which is used in Russia for the treatment of gynecological conditions and as a method of birth control in combination with an estrogen. It has also been studied in the treatment of threatened abortion. It has been used in veterinary medicine as well. It has been marketed since at least 1981.

Allenolic acid, or allenoic acid, is a synthetic, nonsteroidal estrogen discovered in 1947 or 1948 that, although studied clinically, was never marketed. It is an open-ring or seco-analogue of steroidal estrogens like estrone and equilenin. The compound was named after Edgar Allen, one of the pioneers in estrogen research. Although described as an estrogen, allenolic acid probably is totally inactive at the receptor, whereas a derivative, allenestrol, is reported to be a potent estrogen. Another derivative of allenolic acid, methallenestril, is also a potent estrogen and, in contrast to allenolic acid and allenestrol, has been marketed.

Dimethyltrienolone is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and 17α-alkylated 19-nortestosterone (nandrolone) derivative which was never marketed for medical use. It has among the highest known affinity of any AAS for the androgen receptors, and has been said to be perhaps the most potent AAS to have ever been developed.

16-Methylene-17α-hydroxyprogesterone acetate is a progestin of the 17α-hydroxyprogesterone group which was never marketed. Given orally, it shows about 2.5-fold the progestogenic activity of parenteral progesterone in animal bioassays. It is a parent compound of the following clinically used progestins:
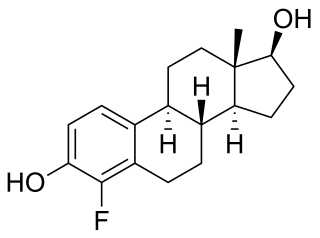
4-Fluoroestradiol (4-FE2) is a synthetic estrogen and a derivative of estradiol which was never marketed. It is specifically the 4-fluoro analogue of estradiol. 4-Fluoroestradiol has 180 ± 43% of the affinity of estradiol for the estrogen receptor of rat uterine cytosol and shows potent uterotrophic activity similar to that of estradiol in mice and rats. It has been labeled with fluorine-18 (18F) for potential use in medical imaging.




















