
Streptococcus is a genus of gram-positive or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales, in the phylum Bacillota. Cell division in streptococci occurs along a single axis, thus when growing they tend to form pairs or chains, which may appear bent or twisted. This differs from staphylococci, which divide along multiple axes, thereby generating irregular, grape-like clusters of cells. Most streptococci are oxidase-negative and catalase-negative, and many are facultative anaerobes.

Group A streptococcal infections are a number of infections with Streptococcus pyogenes, a group A streptococcus (GAS). S. pyogenes is a species of beta-hemolytic Gram-positive bacteria that is responsible for a wide range of infections that are mostly common and fairly mild. If the bacteria enters the bloodstream, the infection can become severe and life-threatening, and is called an invasive GAS (iGAS).

Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause group A streptococcal infection. S. pyogenes is the predominant species harboring the Lancefield group A antigen, and is often called group A Streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) Streptococcus is thus also used.

Streptococcal pharyngitis, also known as streptococcal sore throat, is pharyngitis caused by Streptococcus pyogenes, a gram-positive, group A streptococcus. Common symptoms include fever, sore throat, red tonsils, and enlarged lymph nodes in the front of the neck. A headache and nausea or vomiting may also occur. Some develop a sandpaper-like rash which is known as scarlet fever. Symptoms typically begin one to three days after exposure and last seven to ten days.

Streptococcus agalactiae is a gram-positive coccus with a tendency to form chains. It is a beta-hemolytic, catalase-negative, and facultative anaerobe.

Group B streptococcal infection, also known as Group B streptococcal disease or just Group B strep infection, is the infectious disease caused by the bacterium Streptococcus agalactiae. Streptococcus agalactiae is the most common human pathogen belonging to group B of the Lancefield classification of streptococci—hence the name of group B stretococcal (GBS). Infection with GBS can cause serious illness and sometimes death, especially in newborns, the elderly, and people with compromised immune systems. The most severe form of group B streptococcal disease is neonatal meningitis in infants, which is frequently lethal and can cause permanent neuro-cognitive impairment.
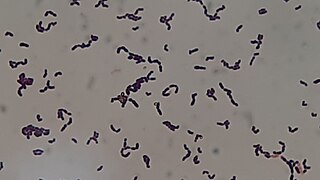
Streptococcus sanguinis, formerly known as Streptococcus sanguis, is a Gram-positive facultative anaerobic coccus species of bacteria and a member of the Viridans Streptococcus group. S. sanguinis is a normal inhabitant of the healthy human mouth where it is particularly found in dental plaque, where it modifies the environment to make it less hospitable for other strains of Streptococcus that cause cavities, such as Streptococcus mutans.

Clobetasone (INN) is a corticosteroid used in dermatology, for treating such skin inflammation as seen in eczema, psoriasis and other forms of dermatitis, and ophthalmology. Topical clobetasone butyrate has shown minimal suppression of the hypothalamic–pituitary–adrenal axis.

Arcanobacterium haemolyticum is a species of bacteria classified as a gram-positive bacillus. It is catalase-negative, facultative anaerobic, beta-hemolytic, and not motile. It has been known to cause head and neck infections, pharyngitis, and sinusitis.
Streptolysins are two homogenous exotoxins from Streptococcus pyogenes. Types include streptolysin O, which is oxygen-labile, and streptolysin S, which is oxygen-stable.
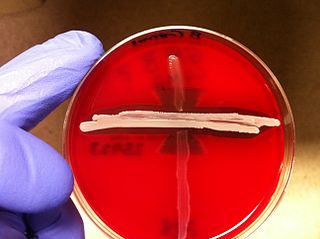
The CAMP test (Christie–Atkins–Munch-Petersen) is a test to identify group B β-hemolytic streptococci based on their formation of a substance, CAMP factor, that enlarges the area of hemolysis formed by the β-hemolysin elaborated from Staphylococcus aureus.

Streptococcus canis is a group G beta-hemolytic species of Streptococcus. It was first isolated in dogs, giving the bacterium its name. These bacteria are characteristically different from Streptococcus dysgalactiae, which is a human-specific group G species that has a different phenotypic chemical composition. S. canis is important to the skin and mucosal health of cats and dogs, but under certain circumstances, these bacteria can cause opportunistic infections. These infections were known to afflict dogs and cats prior to the formal description of the species in Devriese et al., 1986. However, additional studies revealed cases of infection in other mammal species, including cattle and even humans. Instances of mortality from S. canis in humans are very low with only a few reported cases, while actual instances of infection may be underreported due to mischaracterizations of the bacteria as S. dysgalactiae. This species, in general, is highly susceptible to antibiotics, and plans to develop a vaccine to prevent human infections are currently being considered.

Streptococcus dysgalactiae is a gram positive, beta-haemolytic, coccal bacterium belonging to the family Streptococcaceae. It is capable of infecting both humans and animals, but is most frequently encountered as a commensal of the alimentary tract, genital tract, or less commonly, as a part of the skin flora. The clinical manifestations in human disease range from superficial skin-infections and tonsillitis, to severe necrotising fasciitis and bacteraemia. The incidence of invasive disease has been reported to be rising. Several different animal species are susceptible to infection by S. dysgalactiae, but bovine mastitis and infectious arthritis in lambs have been most frequently reported.

Streptococcus iniae is a species of Gram-positive, sphere-shaped bacterium belonging to the genus Streptococcus. Since its isolation from an Amazon freshwater dolphin in the 1970s, S. iniae has emerged as a leading fish pathogen in aquaculture operations worldwide, resulting in over US$100M in annual losses. Since its discovery, S. iniae infections have been reported in at least 27 species of cultured or wild fish from around the world. Freshwater and saltwater fish including tilapia, red drum, hybrid striped bass, and rainbow trout are among those susceptible to infection by S. iniae. Infections in fish manifest as meningoencephalitis, skin lesions, and septicemia.
Perianal cellulitis, also known as perianitis or perianal streptococcal dermatitis, is a bacterial infection affecting the lower layers of the skin (cellulitis) around the anus. It presents as bright redness in the skin and can be accompanied by pain, difficulty defecating, itching, and bleeding. This disease is considered a complicated skin and soft tissue infection (cSSTI) because of the involvement of the deeper soft tissues.
Bacteriophage T12 is a bacteriophage that infects Streptococcus pyogenes bacteria. It is a proposed species of the family Siphoviridae in the order Caudovirales also known as tailed viruses. It converts a harmless strain of bacteria into a virulent strain. It carries the speA gene which codes for erythrogenic toxin A. speA is also known as streptococcal pyogenic exotoxin A, scarlet fever toxin A, or even scarlatinal toxin. Note that the name of the gene "speA" is italicized; the name of the toxin "speA" is not italicized. Erythrogenic toxin A converts a harmless, non-virulent strain of Streptococcus pyogenes to a virulent strain through lysogeny, a life cycle which is characterized by the ability of the genome to become a part of the host cell and be stably maintained there for generations. Phages with a lysogenic life cycle are also called temperate phages. Bacteriophage T12, proposed member of family Siphoviridae including related speA-carrying bacteriophages, is also a prototypic phage for all the speA-carrying phages of Streptococcus pyogenes, meaning that its genome is the prototype for the genomes of all such phages of S. pyogenes. It is the main suspect as the cause of scarlet fever, an infectious disease that affects small children.
In molecular biology, the FasX small RNA (fibronectin/fibrinogen-binding/haemolytic-activity/streptokinase-regulator-X) is a non-coding small RNA (sRNA) produced by all group A Streptococcus. FasX has also been found in species of group D and group G Streptococcus. FasX regulates expression of secreted virulence factor streptokinase (SKA), encoded by the ska gene. FasX base pairs to the 5' end of the ska mRNA, increasing the stability of the mRNA, resulting in elevated levels of streptokinase expression. FasX negatively regulates the expression of pili and fibronectin-binding proteins on the bacterial cell surface. It binds to the 5' untranslated region of genes in the FCT-region in a serotype-specific manner, reducing the stability of and inhibiting translation of the pilus biosynthesis operon mRNA by occluding the ribosome-binding site through a simple Watson-Crick base-pairing mechanism.

Lancefield grouping is a system of classification that classifies catalase-negative Gram-positive cocci based on the carbohydrate composition of bacterial antigens found on their cell walls. The system, created by Rebecca Lancefield, was historically used to organize the various members of the family Streptococcaceae, which includes the genera Lactococcus and Streptococcus, but now is largely superfluous due to explosive growth in the number of streptococcal species identified since the 1970s. However, it has retained some clinical usefulness even after the taxonomic changes, and as of 2018, Lancefield designations are still often used to communicate medical microbiological test results.

Granada medium is a selective and differential culture medium designed to selectively isolate Streptococcus agalactiae and differentiate it from other microorganisms. Granada Medium was developed by Manuel Rosa-Fraile et al. at the Service of Microbiology in the Hospital Virgen de las Nieves in Granada (Spain).
Streptococcosis is an infectious disease caused by bacteria of the genus Steptococcus. This disease is most common among horses, guinea pigs, dogs, cats, and fish with symptoms varying based on the streptococcal species involved. In humans, this disease typically involves a throat infection and is called streptococcal pharyngitis or strep throat.


















