Isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:
A tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 (aldotetroses) or a ketone functional group in position 2 (ketotetroses).
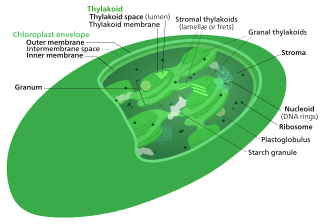
The Calvin cycle,light-independent reactions, bio synthetic phase,dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.
Dihydroxyacetone phosphate (DHAP, also glycerone phosphate in older texts) is the anion with the formula HOCH2C(O)CH2OPO32-. This anion is involved in many metabolic pathways, including the Calvin cycle in plants and glycolysis. It is the phosphate ester of dihydroxyacetone.

Ribulose is a ketopentose — a monosaccharide containing five carbon atoms, and including a ketone functional group. It has chemical formula C5H10O5. Two enantiomers are possible, d-ribulose and l-ribulose. d-Ribulose is the diastereomer of d-xylulose.
Methylotrophs are a diverse group of microorganisms that can use reduced one-carbon compounds, such as methanol or methane, as the carbon source for their growth; and multi-carbon compounds that contain no carbon-carbon bonds, such as dimethyl ether and dimethylamine. This group of microorganisms also includes those capable of assimilating reduced one-carbon compounds by way of carbon dioxide using the ribulose bisphosphate pathway. These organisms should not be confused with methanogens which on the contrary produce methane as a by-product from various one-carbon compounds such as carbon dioxide. Some methylotrophs can degrade the greenhouse gas methane, and in this case they are called methanotrophs. The abundance, purity, and low price of methanol compared to commonly used sugars make methylotrophs competent organisms for production of amino acids, vitamins, recombinant proteins, single-cell proteins, co-enzymes and cytochromes.
The L-arabinose operon, also called the ara or araBAD operon, is an operon required for the breakdown of the five-carbon sugar L-arabinose in Escherichia coli. The L-arabinose operon contains three structural genes: araB, araA, araD, which encode for three metabolic enzymes that are required for the metabolism of L-arabinose. AraB (ribulokinase), AraA, AraD produced by these genes catalyse conversion of L-arabinose to an intermediate of the pentose phosphate pathway, D-xylulose-5-phosphate.
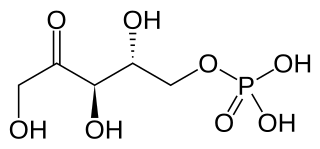
Ribulose 5-phosphate is one of the end-products of the pentose phosphate pathway. It is also an intermediate in the Calvin cycle.

D-Xylulose 5-phosphate (D-xylulose-5-P) is an intermediate in the pentose phosphate pathway. It is a ketose sugar formed from ribulose-5-phosphate by ribulose-5-phosphate epimerase. In the non-oxidative branch of the pentose phosphate pathway, xylulose-5-phosphate acts as a donor of two-carbon ketone groups in transketolase reactions.

Phosphopentose epimerase encoded by the RPE gene is a metalloprotein that catalyzes the interconversion between D-ribulose 5-phosphate and D-xylulose 5-phosphate.
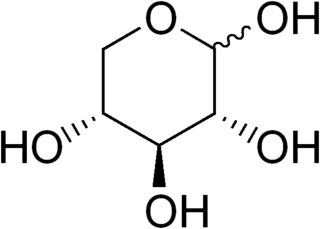
D-Xylose is a five-carbon aldose that can be catabolized or metabolized into useful products by a variety of organisms.
In enzymology, a phosphogluconate dehydrogenase (decarboxylating) (EC 1.1.1.44) is an enzyme that catalyzes the chemical reaction

In enzymology, a L-ribulose-5-phosphate 4-epimerase is an enzyme that catalyzes the interconversion of ribulose 5-phosphate and xylulose 5-phosphate in the oxidative phase of the Pentose phosphate pathway.

Ribose-5-phosphate isomerase (Rpi) encoded by the RPIA gene is an enzyme that catalyzes the conversion between ribose-5-phosphate (R5P) and ribulose-5-phosphate (Ru5P). It is a member of a larger class of isomerases which catalyze the interconversion of chemical isomers. It plays a vital role in biochemical metabolism in both the pentose phosphate pathway and the Calvin cycle. The systematic name of this enzyme class is D-ribose-5-phosphate aldose-ketose-isomerase.
The enzyme 3-dehydro-L-gulonate-6-phosphate decarboxylase (EC 4.1.1.85) catalyzes the chemical reaction
In enzymology, a L-xylulokinase is an enzyme that catalyzes the chemical reaction
Phosphoribulokinase (PRK) (EC 2.7.1.19) is an essential photosynthetic enzyme that catalyzes the ATP-dependent phosphorylation of ribulose 5-phosphate (RuP) into ribulose 1,5-bisphosphate (RuBP), both intermediates in the Calvin Cycle. Its main function is to regenerate RuBP, which is the initial substrate and CO2-acceptor molecule of the Calvin Cycle. PRK belongs to the family of transferase enzymes, specifically those transferring phosphorus-containing groups (phosphotransferases) to an alcohol group acceptor. Along with ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCo), phosphoribulokinase is unique to the Calvin Cycle. Therefore, PRK activity often determines the metabolic rate in organisms for which carbon fixation is key to survival. Much initial work on PRK was done with spinach leaf extracts in the 1950s; subsequent studies of PRK in other photosynthetic prokaryotic and eukaryotic organisms have followed. The possibility that PRK might exist was first recognized by Weissbach et al. in 1954; for example, the group noted that carbon dioxide fixation in crude spinach extracts was enhanced by the addition of ATP. The first purification of PRK was conducted by Hurwitz and colleagues in 1956.
ATP + Mg2+ - D-ribulose 5-phosphate ADP + D-ribulose 1,5-bisphosphate

Ribulose-phosphate 3-epimerase is an enzyme that in humans is encoded by the RPE gene.
The PTS L-Ascorbate (L-Asc) Family includes porters specific for L-ascorbate, and is part of the PTS-AG superfamily. A single PTS permease of the L-Asc family of PTS permeases has been functionally characterized. This is the SgaTBA system, renamed UlaABC by Yew and Gerlt.











