Related Research Articles

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.
A regenerative fuel cell or reverse fuel cell (RFC) is a fuel cell run in reverse mode, which consumes electricity and chemical B to produce chemical A. By definition, the process of any fuel cell could be reversed. However, a given device is usually optimized for operating in one mode and may not be built in such a way that it can be operated backwards. Standard fuel cells operated backwards generally do not make very efficient systems unless they are purpose-built to do so as with high-pressure electrolysers, regenerative fuel cells, solid-oxide electrolyser cells and unitized regenerative fuel cells.
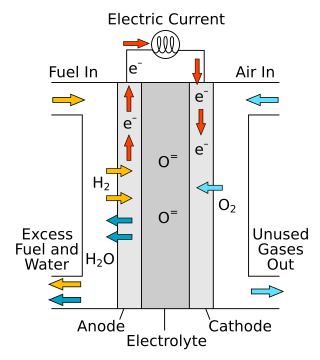
A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.
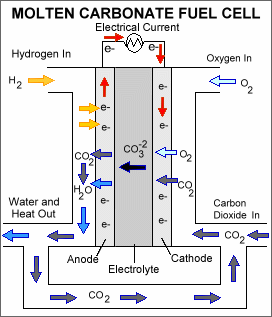
Molten-carbonate fuel cells (MCFCs) are high-temperature fuel cells that operate at temperatures of 600 °C and above.

High-temperature electrolysis is a technology for producing hydrogen from water at high temperatures or other products, such as iron or carbon nanomaterials, as higher energy lowers needed electricity to split molecules and opens up new, potentially better electrolytes like molten salts or hydroxides. Unlike electrolysis at room temperature, HTE operates at elevated temperature ranges depending on the thermal capacity of the material. Because of the detrimental effects of burning fossil fuels on humans and the environment, HTE has become a necessary alternative and efficient method by which hydrogen can be prepared on a large scale and used as fuel. The vision of HTE is to move towards decarbonization in all economic sectors. The material requirements for this process are: the heat source, the electrodes, the electrolyte, the electrolyzer membrane, and the source of electricity.

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.

A protonic ceramic fuel cell or PCFC is a fuel cell based around a ceramic, solid, electrolyte material as the proton conductor from anode to cathode. These fuel cells produce electricity by removing an electron from a hydrogen atom, pushing the charged hydrogen atom through the ceramic membrane, and returning the electron to the hydrogen on the other side of the ceramic membrane during a reaction with oxygen. The reaction of many proposed fuels in PCFCs produce electricity and heat, the latter keeping the device at a suitable temperature. Efficient proton conductivity through most discovered ceramic electrolyte materials require elevated operational temperatures around 600-700 degrees Celsius, however intermediate temperature (200-400 degrees Celsius) ceramic fuel cells and lower temperature alternative are an active area of research. In addition to hydrogen gas, the ability to operate at intermediate and high temperatures enables the use of a variety of liquid hydrogen carrier fuels, including: ammonia, and methane. The technology shares the thermal and kinetic advantages of high temperature molten carbonate and solid oxide fuel cells, while exhibiting all of the intrinsic benefits of proton conduction in proton-exchange membrane fuel cells (PEMFC) and phosphoric acid fuel cells (PAFC). PCFCs exhaust water at the cathode and unused fuel, fuel reactant products and fuel impurities at the anode. Common chemical compositions of the ceramic membranes are barium zirconate (BaZrO3), barium cerate (BaCeO3), caesium dihydrogen phosphate (CsH2PO4), and complex solid solutions of those materials with other ceramic oxides. The acidic oxide ceramics are sometimes broken into their own class of protonic ceramic fuel cells termed "solid acid fuel cells".
Direct-ethanol fuel cells or DEFCs are a category of fuel cell in which ethanol is fed directly into the cell. They have been used as a model to investigate a range of fuel cell concepts including the use of PEM.

Lanthanum strontium manganite (LSM or LSMO) is an oxide ceramic material with the general formula La1−xSrxMnO3, where x describes the doping level.
A Direct Carbon Fuel Cell (DCFC) is a fuel cell that uses a carbon rich material as a fuel such as bio-mass or coal. The cell produces energy by combining carbon and oxygen, which releases carbon dioxide as a by-product. It is also called coal fuel cells (CFCs), carbon-air fuel cells (CAFCs), direct carbon/coal fuel cells (DCFCs), and DC-SOFC.

A solid oxide electrolyzer cell (SOEC) is a solid oxide fuel cell that runs in regenerative mode to achieve the electrolysis of water by using a solid oxide, or ceramic, electrolyte to produce hydrogen gas and oxygen. The production of pure hydrogen is compelling because it is a clean fuel that can be stored, making it a potential alternative to batteries, methane, and other energy sources. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to thermochemical and photocatalytic methods.
The Stalker was a hand-launched, electrically powered unmanned aerial vehicle designed and built by Edge Autonomy and originally sold by Lockheed Martin Skunk Works for an unspecified customer, presumably United States Special Operations Command. It was used for military applications, such as providing intelligence, surveillance, and target acquisition.
Lanthanum manganite is an inorganic compound with the formula LaMnO3, often abbreviated as LMO. Lanthanum manganite is formed in the perovskite structure, consisting of oxygen octahedra with a central Mn atom. The cubic perovskite structure is distorted into an orthorhombic structure by a strong Jahn–Teller distortion of the oxygen octahedra.
A complex oxide is a chemical compound that contains oxygen and at least two other elements. Complex oxide materials are notable for their wide range of magnetic and electronic properties, such as ferromagnetism, ferroelectricity, and high-temperature superconductivity. These properties often come from their strongly correlated electrons in d or f orbitals.
Lanthanum cobaltite is a perovskite with chemical formula LaCoO3. As a solid, the structure LaCoO3, will exist as rhombohedral material at room temperature with ferroelastic properties; though at temperatures above ~900 °C a phase transition to a cubic lattice occurs.

Mixed conductors, also known as mixed ion-electron conductors(MIEC), are a single-phase material that has significant conduction ionically and electronically. Due to the mixed conduction, a formally neutral species can transport in a solid and therefore mass storage and redistribution are enabled. Mixed conductors are well known in conjugation with high-temperature superconductivity and are able to capacitate rapid solid-state reactions.
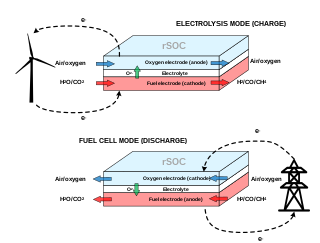
A reversible solid oxide cell (rSOC) is a solid-state electrochemical device that is operated alternatively as a solid oxide fuel cell (SOFC) and a solid oxide electrolysis cell (SOEC). Similarly to SOFCs, rSOCs are made of a dense electrolyte sandwiched between two porous electrodes. Their operating temperature ranges from 600°C to 900°C, hence they benefit from enhanced kinetics of the reactions and increased efficiency with respect to low-temperature electrochemical technologies.
A process related to the sol-gel route is the Pechini, or liquid mix, process. An aqueous solution of suitable oxides or salts is mixed with an alpha-hydroxycarboxylic acid such as citric acid. Chelation, or the formation of complex ring-shaped compounds around the metal cations, takes place in the solution. A polyhydroxy alcohol is then added, and the liquid is heated to 150–250 °C (300–480 °F) to allow the chelates to polymerize, or form large, cross-linked networks. As excess water is removed by heating, a solid polymeric resin results. Eventually, at still higher temperatures of 500–900 °C (930–1,650 °F), the resin is decomposed or charred, and ultimately a mixed oxide is obtained. Particle size is extremely small, typically 20 to 50 nanometres, with intimate mixing taking place on the atomic scale.
References
- ↑ Chang, Hun-Chieh; Tsai, Dah-Shyang; Chung, Wen-Hung; Huang, Ying-Sheng; Le, Minh-Vien (27 April 2009). "A ceria layer as diffusion barrier between LAMOX and lanthanum strontium cobalt ferrite along with the impedance analysis". Solid State Ionics. 180 (4–5): 412–417. doi:10.1016/j.ssi.2009.01.018.
- 1 2 Kulkarni, A.; Ciacchi, F.T.; Giddey, S.; Munnings, C.; Badwal, S.P.S.; Kimpton, J.A.; Fini, D. (December 2012). "Mixed ionic electronic conducting perovskite anode for direct carbon fuel cells". International Journal of Hydrogen Energy. 37 (24): 19092–19102. Bibcode:2012IJHE...3719092K. doi:10.1016/j.ijhydene.2012.09.141.
- ↑ Badwal, SPS; Giddey, S; Munnings, C; Kulkarni, A (2014). "Review of Progress in High Temperature Solid Oxide Fuel Cells". Journal of the Australian Ceramics Society. 50 (1).
- ↑ Munnings, C.; Kulkarni, A.; Giddey, S.; Badwal, S.P.S. (August 2014). "Biomass to power conversion in a direct carbon fuel cell". International Journal of Hydrogen Energy. 39 (23): 12377–12385. Bibcode:2014IJHE...3912377M. doi:10.1016/j.ijhydene.2014.03.255.
- ↑ "Ceramic Tubes Could Cut Greenhouse Gas Emissions From Power Stations". ScienceDaily. Retrieved 2020-10-08.