Related Research Articles

Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

The enzyme phenylalanine racemase is the enzyme that acts on amino acids and derivatives. It activates both the L & D stereo isomers of phenylalanine to form L-phenylalanyl adenylate and D-phenylalanyl adenylate, which are bound to the enzyme. These bound compounds are then transferred to the thiol group of the enzyme followed by conversion of its configuration, the D-isomer being the more favorable configuration of the two, with a 7 to 3 ratio between the two isomers. The racemisation reaction of phenylalanine is coupled with the highly favorable hydrolysis of adenosine triphosphate (ATP) to adenosine monophosphate (AMP) and pyrophosphate (PP), thermodynamically allowing it to proceed. This reaction is then drawn forward by further hydrolyzing PP to inorganic phosphate (Pi), via Le Chatelier's principle.
The lactate racemase enzyme (Lar) interconverts the D- and L-enantiomers of lactic acid. It is classified under the isomerase, racemase, epimerase, and enzyme acting on hydroxyl acids and derivatives classes of enzymes. It is found in certain halophilic archaea, such as Haloarcula marismortui, and in a few species of bacteria, such as several Lactobacillus species including Lactobacillus sakei, Lactobacillus curvatus, and Lactobacillus plantarum, as well as in non-lactic acid bacteria such as Clostridium beijerinckii. The gene encoding lactate racemase in L. plantarum was identified as larA and shown to be associated with a widespread maturation system involving larB, larC1, larC2, and larE. The optimal pH for its activity is 5.8-6.2 in L. sakei.

Methylmalonyl CoA epimerase is an enzyme involved in fatty acid catabolism that is encoded in human by the "MCEE" gene located on chromosome 2. It is routinely and incorrectly labeled as "methylmalonyl-CoA racemase". It is not a racemase because the CoA moiety has 5 other stereocenters.
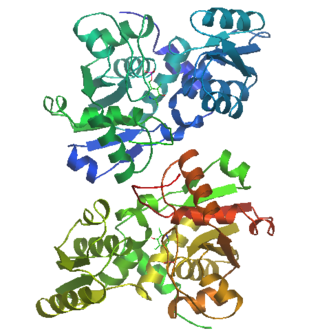
Serine racemase is the first racemase enzyme in human biology to be identified. This enzyme converts L-serine to its enantiomer form, D-serine. D-serine acts as a neuronal signaling molecule by activating NMDA receptors in the brain.
In enzymology, a 2-aminohexano-6-lactam racemase is an enzyme that catalyzes the chemical reaction

In enzymology, an alanine racemase is an enzyme that catalyzes the chemical reaction
In enzymology, an amino-acid racemase is an enzyme that catalyzes the chemical reaction
In enzymology, an arginine racemase is an enzyme that catalyzes the chemical reaction
In enzymology, an aspartate racemase is an enzyme that catalyzes the following chemical reaction:
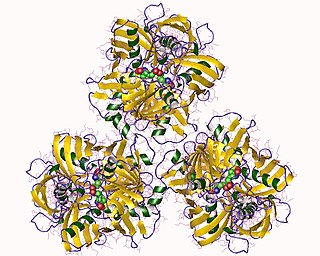
In enzymology, a diaminopimelate epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, glutamate racemase is an enzyme that catalyzes the chemical reaction
In enzymology, a methionine racemase is an enzyme that catalyzes the chemical reaction
In enzymology, an ornithine racemase is an enzyme that catalyzes the chemical reaction
In enzymology, a proline racemase is an enzyme that catalyzes the chemical reaction
In enzymology, a threonine racemase is an enzyme that catalyzes the chemical reaction

The enzyme diaminopimelate decarboxylase (EC 4.1.1.20) catalyzes the cleavage of carbon-carbon bonds in meso 2,6 diaminoheptanedioate to produce CO2 and L-lysine, the essential amino acid. It employs the cofactor pyridoxal phosphate, also known as PLP, which participates in numerous enzymatic transamination, decarboxylation and deamination reactions.
Hydantoin racemase is an enzyme with systematic name D-5-monosubstituted-hydantoin racemase. This enzyme catalyses the following chemical reaction
References
- Huang HT (1960). "Pat". Chem. Abstr. 54: 20073.