
N-Acetylmannosamine is a hexosamine monosaccharide. It is a neutral, stable naturally occurring compound. N-Acetylmannosamine is also known as N-Acetyl-D-mannosamine monohydrate,, N-Acetyl-D-mannosamine which can be abbreviated to ManNAc or, less commonly, NAM). ManNAc is the first committed biological precursor of N-acetylneuraminic acid. Sialic acids are the negatively charged, terminal monosaccharides of carbohydrate chains that are attached to glycoproteins and glycolipids (glycans).

Phosphopentose epimerase encoded by the RPE gene is a metalloprotein that catalyzes the interconversion between D-ribulose 5-phosphate and D-xylulose 5-phosphate.

Methylmalonyl CoA epimerase is an enzyme involved in fatty acid catabolism that is encoded in human by the "MCEE" gene located on chromosome 2. It is routinely and incorrectly labeled as "methylmalonyl-CoA racemase". It is not a racemase because the CoA moiety has 5 other stereocenters.
In enzymology, a N-acylmannosamine 1-dehydrogenase (EC 1.1.1.233) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acylhexosamine oxidase (EC 1.1.3.29) is an enzyme that catalyzes the chemical reaction
In enzymology, a diaminopimelate epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acylglucosamine-6-phosphate 2-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-N-acetylglucosamine 2-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-N-acetylglucosamine 4-epimerase is an enzyme that catalyzes the chemical reaction

In enzymology, N-acetylglucosamine-6-phosphate deacetylase (EC 3.5.1.25), also known as GlcNAc-6-phosphate deacetylase or NagA, is an enzyme that catalyzes the deacetylation of N-acetylglucosamine-6-phosphate (GlcNAc-6-P) to glucosamine-6-phosphate (GlcN-6-P):
In enzymology, a N-acylneuraminate-9-phosphate synthase (EC 2.5.1.57) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acylmannosamine kinase is an enzyme that catalyzes the chemical reaction

N-acylglucosamine 2-epimerase is an enzyme that in humans is encoded by the RENBP gene.
UDP-3-O-(3-hydroxymyristoyl)glucosamine N-acyltransferase is an enzyme with systematic name (3R)-3-hydroxymyristoyl-(acyl-carrier protein):UDP-3-O-( -3-hydroxymyristoyl)-alpha-D-glucosamine N-acetyltransferase. This enzyme catalyses the following chemical reaction
UDP-N-acetylglucosamine 2-epimerase (hydrolysing) (EC 3.2.1.183, UDP-N-acetylglucosamine 2-epimerase, GNE (gene), siaA (gene), neuC (gene)) is an enzyme with systematic name UDP-N-acetyl-alpha-D-glucosamine hydrolase (2-epimerising). This enzyme catalyses the following chemical reaction

Epimerox is an experimental broad-spectrum antibiotic compound being developed by scientists at the Rockefeller University and Astex Pharmaceuticals. It is a small molecule inhibitor compound that blocks the activity of the enzyme UDP-N-acetylglucosamine 2-epimerase, an epimerase enzyme that is called 2-epimerase for short.
2-Epimerase can refer one of to several enzymes:
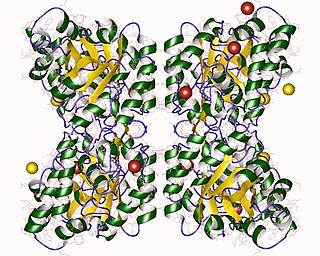
4-Hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), dapA (gene)) is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinate-forming). This enzyme catalyses the following chemical reaction
N-acetyl-β-d-glucosaminidase(EC 3.2.1.30; EC 3.2.1.52) is a mesophilic hydrolase that specifically hydrolyzes N-acetyl-glucosides. The enzyme is found across a wide variety of marine and terrestrial creatures with the primary function of breaking down oligosaccharides in the presence of water. One of the primary functions of the enzyme is to target and hydrolyze oligosaccharides containing chitin. In this chitinase function, the enzyme contributes to the ability of many organisms to break down chitin-containing molecules and subsequently digest or re-uptake environmental chitin, carbon, or nitrogen. The enzyme's crystal structure varies slightly across organisms, but is characterized by three or four domains with one active site. Across proteins, the active site entails an α-β barrel with either an arginine or tryptophan residues in the barrel pocket to bind incoming substrate.












